通过掺杂过渡金属操纵二氧化锰中的 d 电子态,促进海水直接电解
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
随着淡水日益稀缺,海水电解在制氢方面具有巨大潜力。然而,阳极氧进化反应(OER)的缓慢动力学限制了法拉第效率,而且海水中高浓度的氯离子会导致竞争反应,产生氯或次氯酸盐等高腐蚀性副产品。二氧化锰在酸性条件下表现出卓越的稳定性,其性能明显优于最先进的 OER 贵金属催化剂 RuO2。相反,MnO2 的 OER 活性却远不如 RuO2。在本研究中,我们设计了 13 种掺杂过渡金属的 γ-MnO2 催化剂(TM-MnO2),并基于密度泛函理论筛选出 6 种具有高活性和高选择性的潜在催化剂,用于直接电解海水。我们深入研究了活性的来源以及 pH 值对选择性的影响。我们的研究表明,γ-MnO2 中分散的 TM 增强了 TM-O 键的高共价性,从而引发了晶格氧机制(LOM),而不是传统的吸附剂进化机制(AEM)。值得注意的是,TM-O 键的集成晶体轨道汉密尔顿群(ICOHP)与 TM d 带中心(εd)之间存在火山型关系,揭示了掺杂策略可以通过调节 TM εd 来操纵 TM-O 键的共价性,从而调节其活性。此外,我们还通过构建 Pourbaix 图确定了催化剂在一定电位 U 和 pH 值范围内的稳定性。最后,我们验证了掺杂钼的γ-MnO2 在抑制氯氧化反应(ClOR)方面表现出卓越的 OER 性能(过电位为 0.27 V)和高选择性。这项研究为设计和开发先进的 OER 催化剂提供了理论依据,这种催化剂可同时抑制直接电解海水中的 ClOR。本文章由计算机程序翻译,如有差异,请以英文原文为准。
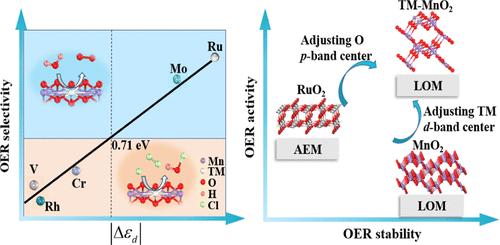
Manipulating d-Electronic States via Transition Metal Doping in MnO2 to Boost Direct Seawater Electrolysis
With the increasing scarcity of fresh water, seawater electrolysis holds great potential for hydrogen production. However, the slow kinetics of anodic oxygen evolution reaction (OER) limits the Faraday efficiency, and the high concentration of chloride ions in seawater leads to the competitive reaction that produces highly corrosive byproducts such as chlorine or hypochlorite. MnO2 has demonstrated exceptional stability under acidic conditions, remarkably outperforming state-of-the-art OER noble metal catalyst, RuO2. Conversely, the OER activity of MnO2 is far inferior to that of RuO2. In this study, we have designed 13 transition metal-doped γ-MnO2 catalysts (TM-MnO2) and screened 6 potential catalysts for direct seawater electrolysis with high activity and selectivity based on density functional theory. We thoroughly investigated the origin of activity and the effect of pH on selectivity. Our work demonstrates that the dispersed TM in γ-MnO2 enhances the high covalency of the TM–O bond, thereby triggering the lattice oxygen mechanism (LOM) instead of the traditional adsorbate evolution mechanism (AEM). Notably, there is a volcano-type relationship between integrated crystal orbital Hamilton population (ICOHP) of the TM–O bond and the TM d-band center (εd), unveiling that the doping strategy can manipulate the covalency of the TM–O bond through tuning the TM εd, thereby regulating the activity. Moreover, we determine the stability of the catalysts at a range of potential U and pH values through constructing the Pourbaix diagrams. Finally, we validate that γ-MnO2 doped with Mo exhibits superior OER performance with an overpotential of 0.27 V and high selectivity in suppressing the chloride oxidation reaction (ClOR). This study provides theoretical insights into the design and development of advanced OER catalysts, which can simultaneously suppress ClOR for direct seawater electrolysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


