多脯氨酸 II 螺旋束中的氢键模式和合作性
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氢键合作性(HBC)在稳定由α螺旋和β片层(最常见的二级结构)构建的蛋白质组装方面发挥着重要作用。然而,HBC 是否存在于其他类型的蛋白质二级结构(如多脯氨酸 II(PPII)螺旋)中仍有待探索。这一点很耐人寻味,因为作为组装模块的 PPII 系统正在多个领域不断涌现。在这里,我们结合使用计算化学工具和分子建模,并辅以实验观察结果,描述了 PPII 螺旋束中存在的独特 H 键模式,并确定 HBC 可稳定分子间 PPII 螺旋,正如在淀粉样纤维等其他蛋白质组装体中看到的那样。除了规范的 CO-HN H 键中的合作性相互作用外,我们还发现非规范的 CO-HαCα H 键中的类似相互作用也与富含甘氨酸的 PPII 束有关,从而弥补了甘氨酸残基无法形成疏水核心的缺陷。我们的研究结果为这些束的组装提供了一种机理解释。氢键合作性(HBC)在由α螺旋和β片所构建的蛋白质组装的稳定性中起着重要作用,然而,HBC是否也存在于多脯氨酸II(PPII)螺旋中仍是未知数。在本文中,作者利用计算化学工具和分子建模证明了 HBC 能稳定分子间的 PPII 螺旋,并得到了实验观察的证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
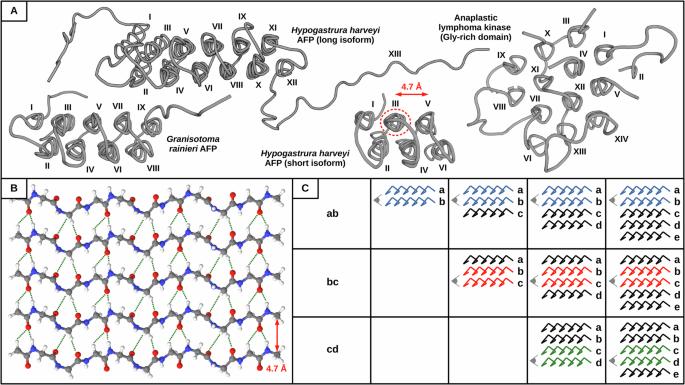
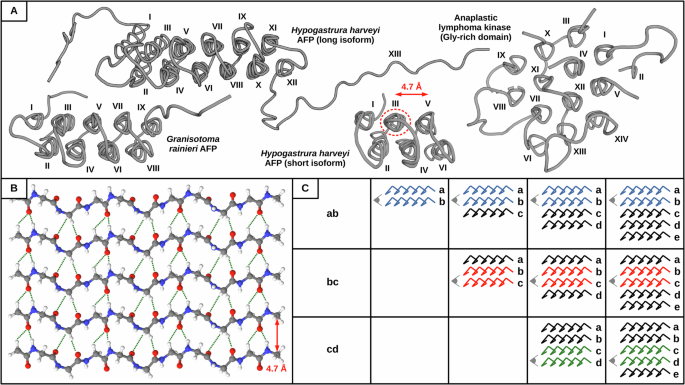
Hydrogen bonding patterns and cooperativity in polyproline II helical bundles
Hydrogen bond cooperativity (HBC) plays an important role in stabilizing protein assemblies built by α-helices and β-sheets, the most common secondary structures. However, whether HBC exists in other types of protein secondary structures such as polyproline II (PPII) helices remains unexplored. This is intriguing, since PPII systems as assembling blocks are continuously emerging across multiple fields. Here, using a combination of computational chemistry tools and molecular modeling corroborated by experimental observables, we characterize the distinct H-bonding patterns present in PPII helical bundles and establish that HBC stabilizes intermolecular PPII helices as seen in other protein assemblies such as amyloid fibrils. In addition to cooperative interactions in canonical CO···HN H-bonds, we show that analogous interactions in non-canonical CO···HαCα H-bonds are relevant in Gly-rich PPII bundles, thus compensating for the inability of glycine residues to create hydrophobic cores. Our results provide a mechanistic explanation for the assembly of these bundles. Hydrogen bond cooperativity (HBC) plays an important role in the stability of protein assemblies built by α-helices and β-sheets, however, it remains unknown whether HBC also exists in polyproline II (PPII) helices. Here, the authors show that HBC stabilizes intermolecular PPII helices using computational chemistry tools and molecular modeling corroborated by experimental observations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


