光促进 AIBN 催化芳基烯烃氧化裂解为芳基酮
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究开发了一种以分子氧为氧化剂、由芳基烯催化的 2,2-偶氮双(异丁腈)(AIBN)光催化合成芳基酮的简便方法。以中等至良好的产率获得了一系列芳基酮。新方案的特点是条件温和,底物范围相对较广。该反应以良好的收率成功用于合成非诺贝特。根据以前的文献和目前的实验结果,提出了一种合理的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
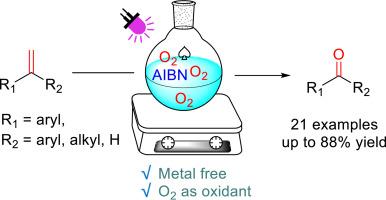
Light-promoted AIBN-catalyzed oxidative cleavage of aryl olefins to aryl ketones
A convenient light-promoted 2,2-azobis(isobutyronitrile) (AIBN)-catalyzed synthesis of aryl ketone from aryl alkenes with molecular oxygen as the oxidant was developed. A series of aryl ketone were obtained in moderate to good yields. The new protocol features mild conditions and relatively broad substrate scope. The reaction was successfully applied for the synthesis of Fenofibrate in good yield. Based on previous documents and current experimental results, a plausible mechanism is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


