父代运动通过α-Klotho/Keap1途径诱导暴露于高脂肪饮食的后代骨骼肌的抗氧化防御能力,而不会改变端粒长度。
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
尽管之前的研究表明,祖先的生活方式可以增强暴露于肥胖饮食的后代的代谢健康,但这些氧化还原状态的积极影响与端粒长度之间的具体联系尚不清楚。我们研究了父亲阻力训练(RT)对骨骼肌应激反应信号传导和端粒平衡相关途径的影响。这项研究涵盖了长期暴露于标准饮食(24 周)和高脂饮食(HFD)的父亲和第一代小鼠。Wistar 大鼠的父亲被随机分为静坐型和训练型(8 周抗阻力训练)。后代由久坐不动的雌鼠交配获得。断奶后,雄性后代被分为四组:静坐或训练父亲的后代,暴露于对照饮食或高纤维食物。对腓肠肌进行反转录-定量聚合酶链反应、免疫印迹、酶联免疫吸附试验和电子顺磁共振波谱分析。RT能上调庇护素mRNA水平和抗氧化蛋白,保护父亲的肌肉端粒。相反,高频分解膳食会导致氧化还原平衡失调,这可能是导致久坐不动的父亲的后代端粒缩短的原因之一。受孕前父亲的RT会下调暴露于高氟酸脱氢食物的后代骨骼肌中Kelch样ECH相关蛋白1(Keap1)的mRNA水平,从而推动谷胱甘肽还原酶mRNA水平、Sod1和过氧化氢酶蛋白水平的增加,以缓解ROS的产生。此外,父代运动还能上调α-Klotho蛋白水平,在不改变庇护素mRNA水平和端粒长度的情况下介导抗氧化反应。我们首次深入分析了后代的氧化还原状态似乎与父亲运动的有益作用直接相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
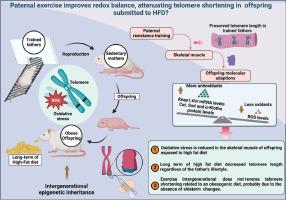
Paternal exercise induces antioxidant defenses by α-Klotho/Keap1 pathways in the skeletal muscle of offspring exposed to a high fat-diet without changing telomere length
Although previous studies demonstrated that the ancestral lifestyle can enhance the metabolic health of offspring exposed to an obesogenic diet, the specific connections between these positive effects in redox state and telomere length are unknown. We investigated the impact of paternal resistance training (RT) on stress-responsive signaling and the pathways involved in telomere homeostasis in skeletal muscle. This investigation encompassed both the fathers and first-generation litter exposed to a long-term standard diet (24 weeks) and high fat diet (HFD). Wistar rats were randomized into sedentary or trained fathers (8 weeks of resistance training). The offspring were obtained by mating with sedentary females. Upon weaning, male offspring were divided into four groups: offspring of sedentary or trained fathers exposed to either a control diet or HFD. The gastrocnemius was prepared for reverse transcription-quantitative polymerase chain reaction, immunoblotting, ELISA, and electron paramagnetic resonance spectroscopy. RT upregulated shelterin mRNA levels and antioxidant protein, preserving muscle telomere in fathers. Conversely, HFD induced a disturbance in the redox balance, which may have contributed to the offspring telomere shortening from sedentary fathers. Preconceptional paternal RT downregulates Kelch-like ECH-associated protein 1 (Keap1) mRNA levels in the skeletal muscle of progeny exposed to HFD, driving an increase in Glutathione reductase mRNA levels, Sod1 and Catalase protein levels to mitigate ROS production. Also, paternal exercise upregulates α-Klotho protein levels, mediating antioxidative responses without altering shelterin mRNA levels and telomere length. We provide the first in-depth analysis that the offspring's redox state seems to be directly associated with the beneficial effects of paternal exercise.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


