求助PDF
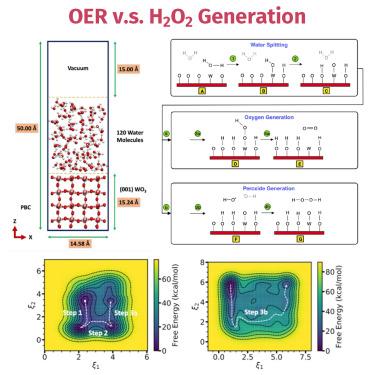
{"title":"(001)-WO3/ 水-液界面水氧化反应的元动力学研究","authors":"Rangsiman Ketkaew, Fabrizio Creazzo, Kevin Sivula, Sandra Luber","doi":"10.1016/j.checat.2024.101085","DOIUrl":null,"url":null,"abstract":"<p>A metadynamics method was used to calculate the free energy surfaces (FESs) of the oxygen evolution reaction (OER). Metadynamics simulation suggests that the oxygen–oxygen (<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mo is=\"true\">&#x2212;</mo><mi mathvariant=\"normal\" is=\"true\">O</mi></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.202ex\" role=\"img\" style=\"vertical-align: -0.351ex;\" viewbox=\"0 -796.9 2779.9 947.9\" width=\"6.457ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-4F\"></use></g><g is=\"true\" transform=\"translate(1000,0)\"><use xlink:href=\"#MJMAIN-2212\"></use></g><g is=\"true\" transform=\"translate(2001,0)\"><use xlink:href=\"#MJMAIN-4F\"></use></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><mi is=\"true\" mathvariant=\"normal\">O</mi><mo is=\"true\">−</mo><mi is=\"true\" mathvariant=\"normal\">O</mi></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mo is=\"true\">−</mo><mi mathvariant=\"normal\" is=\"true\">O</mi></mrow></math></script></span>) bond formation induced by oxidative reactant species and oxygen atoms on the surface is the rate-determining step. Not only bond distances but also extended social permutation invariant (xSPRINT) coordinates and deep autoencoder neural network (DAENN) are used as collective variables (CVs) in metadynamics calculations to characterize the FESs of the studied reactions. The FES calculations using xSPRINT and DAENN CVs show that the formation of <span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msup is=\"true\"><mtext is=\"true\">OH</mtext><mo is=\"true\">&#x2022;</mo></msup></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.202ex\" role=\"img\" style=\"vertical-align: -0.235ex;\" viewbox=\"0 -846.5 1982.9 947.9\" width=\"4.605ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-4F\"></use><use x=\"778\" xlink:href=\"#MJMAIN-48\" y=\"0\"></use></g><g is=\"true\" transform=\"translate(1529,432)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-2219\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msup is=\"true\"><mtext is=\"true\">OH</mtext><mo is=\"true\">•</mo></msup></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><msup is=\"true\"><mtext is=\"true\">OH</mtext><mo is=\"true\">•</mo></msup></mrow></math></script></span> and <span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">H</mi><mn is=\"true\">2</mn></msub><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mn is=\"true\">2</mn></msub></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.432ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -796.9 2436.8 1047.3\" width=\"5.66ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-48\"></use></g><g is=\"true\" transform=\"translate(750,-150)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g><g is=\"true\" transform=\"translate(1204,0)\"><g is=\"true\"><use xlink:href=\"#MJMAIN-4F\"></use></g><g is=\"true\" transform=\"translate(778,-150)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msub is=\"true\"><mi is=\"true\" mathvariant=\"normal\">H</mi><mn is=\"true\">2</mn></msub><msub is=\"true\"><mi is=\"true\" mathvariant=\"normal\">O</mi><mn is=\"true\">2</mn></msub></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">H</mi><mn is=\"true\">2</mn></msub><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mn is=\"true\">2</mn></msub></mrow></math></script></span>, respectively, is more energetically (in terms of the free-energy barrier) favorable than the formation of oxygen gas at the <span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msub is=\"true\"><mtext is=\"true\">WO</mtext><mn is=\"true\">3</mn></msub></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.432ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -796.9 2260.9 1047.3\" width=\"5.251ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-57\"></use><use x=\"1028\" xlink:href=\"#MJMAIN-4F\" y=\"0\"></use></g><g is=\"true\" transform=\"translate(1807,-150)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-33\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msub is=\"true\"><mtext is=\"true\">WO</mtext><mn is=\"true\">3</mn></msub></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><msub is=\"true\"><mtext is=\"true\">WO</mtext><mn is=\"true\">3</mn></msub></mrow></math></script></span> surface/liquid-water interface. It is found that the <span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mo is=\"true\">&#x2212;</mo><mi mathvariant=\"normal\" is=\"true\">O</mi></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.202ex\" role=\"img\" style=\"vertical-align: -0.351ex;\" viewbox=\"0 -796.9 2779.9 947.9\" width=\"6.457ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-4F\"></use></g><g is=\"true\" transform=\"translate(1000,0)\"><use xlink:href=\"#MJMAIN-2212\"></use></g><g is=\"true\" transform=\"translate(2001,0)\"><use xlink:href=\"#MJMAIN-4F\"></use></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><mi is=\"true\" mathvariant=\"normal\">O</mi><mo is=\"true\">−</mo><mi is=\"true\" mathvariant=\"normal\">O</mi></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mo is=\"true\">−</mo><mi mathvariant=\"normal\" is=\"true\">O</mi></mrow></math></script></span> bond formation is sluggish because of the stable electronic state of the surface and that the formation of <span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msup is=\"true\"><mtext is=\"true\">OH</mtext><mo is=\"true\">&#x2022;</mo></msup></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.202ex\" role=\"img\" style=\"vertical-align: -0.235ex;\" viewbox=\"0 -846.5 1982.9 947.9\" width=\"4.605ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-4F\"></use><use x=\"778\" xlink:href=\"#MJMAIN-48\" y=\"0\"></use></g><g is=\"true\" transform=\"translate(1529,432)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-2219\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msup is=\"true\"><mtext is=\"true\">OH</mtext><mo is=\"true\">•</mo></msup></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><msup is=\"true\"><mtext is=\"true\">OH</mtext><mo is=\"true\">•</mo></msup></mrow></math></script></span> and <span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">H</mi><mn is=\"true\">2</mn></msub><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mn is=\"true\">2</mn></msub></mrow></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"2.432ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -796.9 2436.8 1047.3\" width=\"5.66ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"><g is=\"true\"><use xlink:href=\"#MJMAIN-48\"></use></g><g is=\"true\" transform=\"translate(750,-150)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g><g is=\"true\" transform=\"translate(1204,0)\"><g is=\"true\"><use xlink:href=\"#MJMAIN-4F\"></use></g><g is=\"true\" transform=\"translate(778,-150)\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><mrow is=\"true\"><msub is=\"true\"><mi is=\"true\" mathvariant=\"normal\">H</mi><mn is=\"true\">2</mn></msub><msub is=\"true\"><mi is=\"true\" mathvariant=\"normal\">O</mi><mn is=\"true\">2</mn></msub></mrow></math></span></span><script type=\"math/mml\"><math><mrow is=\"true\"><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">H</mi><mn is=\"true\">2</mn></msub><msub is=\"true\"><mi mathvariant=\"normal\" is=\"true\">O</mi><mn is=\"true\">2</mn></msub></mrow></math></script></span> occurs through an energy barrierless process.</p>","PeriodicalId":53121,"journal":{"name":"Chem Catalysis","volume":"30 1","pages":""},"PeriodicalIF":11.5000,"publicationDate":"2024-08-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"A metadynamics study of water oxidation reactions at (001)-WO3/liquid-water interface\",\"authors\":\"Rangsiman Ketkaew, Fabrizio Creazzo, Kevin Sivula, Sandra Luber\",\"doi\":\"10.1016/j.checat.2024.101085\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>A metadynamics method was used to calculate the free energy surfaces (FESs) of the oxygen evolution reaction (OER). Metadynamics simulation suggests that the oxygen–oxygen (<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mo is=\\\"true\\\">&#x2212;</mo><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.202ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.351ex;\\\" viewbox=\\\"0 -796.9 2779.9 947.9\\\" width=\\\"6.457ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(1000,0)\\\"><use xlink:href=\\\"#MJMAIN-2212\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(2001,0)\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">O</mi><mo is=\\\"true\\\">−</mo><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">O</mi></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mo is=\\\"true\\\">−</mo><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi></mrow></math></script></span>) bond formation induced by oxidative reactant species and oxygen atoms on the surface is the rate-determining step. Not only bond distances but also extended social permutation invariant (xSPRINT) coordinates and deep autoencoder neural network (DAENN) are used as collective variables (CVs) in metadynamics calculations to characterize the FESs of the studied reactions. The FES calculations using xSPRINT and DAENN CVs show that the formation of <span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msup is=\\\"true\\\"><mtext is=\\\"true\\\">OH</mtext><mo is=\\\"true\\\">&#x2022;</mo></msup></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.202ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.235ex;\\\" viewbox=\\\"0 -846.5 1982.9 947.9\\\" width=\\\"4.605ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use><use x=\\\"778\\\" xlink:href=\\\"#MJMAIN-48\\\" y=\\\"0\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(1529,432)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-2219\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msup is=\\\"true\\\"><mtext is=\\\"true\\\">OH</mtext><mo is=\\\"true\\\">•</mo></msup></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><msup is=\\\"true\\\"><mtext is=\\\"true\\\">OH</mtext><mo is=\\\"true\\\">•</mo></msup></mrow></math></script></span> and <span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">H</mi><mn is=\\\"true\\\">2</mn></msub><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mn is=\\\"true\\\">2</mn></msub></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.432ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -796.9 2436.8 1047.3\\\" width=\\\"5.66ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-48\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(750,-150)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g><g is=\\\"true\\\" transform=\\\"translate(1204,0)\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(778,-150)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">H</mi><mn is=\\\"true\\\">2</mn></msub><msub is=\\\"true\\\"><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">O</mi><mn is=\\\"true\\\">2</mn></msub></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">H</mi><mn is=\\\"true\\\">2</mn></msub><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mn is=\\\"true\\\">2</mn></msub></mrow></math></script></span>, respectively, is more energetically (in terms of the free-energy barrier) favorable than the formation of oxygen gas at the <span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mtext is=\\\"true\\\">WO</mtext><mn is=\\\"true\\\">3</mn></msub></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.432ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -796.9 2260.9 1047.3\\\" width=\\\"5.251ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-57\\\"></use><use x=\\\"1028\\\" xlink:href=\\\"#MJMAIN-4F\\\" y=\\\"0\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(1807,-150)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-33\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mtext is=\\\"true\\\">WO</mtext><mn is=\\\"true\\\">3</mn></msub></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mtext is=\\\"true\\\">WO</mtext><mn is=\\\"true\\\">3</mn></msub></mrow></math></script></span> surface/liquid-water interface. It is found that the <span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mo is=\\\"true\\\">&#x2212;</mo><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.202ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.351ex;\\\" viewbox=\\\"0 -796.9 2779.9 947.9\\\" width=\\\"6.457ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(1000,0)\\\"><use xlink:href=\\\"#MJMAIN-2212\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(2001,0)\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">O</mi><mo is=\\\"true\\\">−</mo><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">O</mi></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mo is=\\\"true\\\">−</mo><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi></mrow></math></script></span> bond formation is sluggish because of the stable electronic state of the surface and that the formation of <span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msup is=\\\"true\\\"><mtext is=\\\"true\\\">OH</mtext><mo is=\\\"true\\\">&#x2022;</mo></msup></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.202ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.235ex;\\\" viewbox=\\\"0 -846.5 1982.9 947.9\\\" width=\\\"4.605ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use><use x=\\\"778\\\" xlink:href=\\\"#MJMAIN-48\\\" y=\\\"0\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(1529,432)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-2219\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msup is=\\\"true\\\"><mtext is=\\\"true\\\">OH</mtext><mo is=\\\"true\\\">•</mo></msup></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><msup is=\\\"true\\\"><mtext is=\\\"true\\\">OH</mtext><mo is=\\\"true\\\">•</mo></msup></mrow></math></script></span> and <span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">H</mi><mn is=\\\"true\\\">2</mn></msub><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mn is=\\\"true\\\">2</mn></msub></mrow></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"2.432ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -796.9 2436.8 1047.3\\\" width=\\\"5.66ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-48\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(750,-150)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g><g is=\\\"true\\\" transform=\\\"translate(1204,0)\\\"><g is=\\\"true\\\"><use xlink:href=\\\"#MJMAIN-4F\\\"></use></g><g is=\\\"true\\\" transform=\\\"translate(778,-150)\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">H</mi><mn is=\\\"true\\\">2</mn></msub><msub is=\\\"true\\\"><mi is=\\\"true\\\" mathvariant=\\\"normal\\\">O</mi><mn is=\\\"true\\\">2</mn></msub></mrow></math></span></span><script type=\\\"math/mml\\\"><math><mrow is=\\\"true\\\"><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">H</mi><mn is=\\\"true\\\">2</mn></msub><msub is=\\\"true\\\"><mi mathvariant=\\\"normal\\\" is=\\\"true\\\">O</mi><mn is=\\\"true\\\">2</mn></msub></mrow></math></script></span> occurs through an energy barrierless process.</p>\",\"PeriodicalId\":53121,\"journal\":{\"name\":\"Chem Catalysis\",\"volume\":\"30 1\",\"pages\":\"\"},\"PeriodicalIF\":11.5000,\"publicationDate\":\"2024-08-29\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Chem Catalysis\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://doi.org/10.1016/j.checat.2024.101085\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"CHEMISTRY, PHYSICAL\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Chem Catalysis","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.1016/j.checat.2024.101085","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"CHEMISTRY, PHYSICAL","Score":null,"Total":0}
引用次数: 0
引用
批量引用



 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:
