用于胶质母细胞瘤靶向 siRNA 递送和 CRISPR-Cas 基因编辑的聚合物锁定熔融脂质体
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在胶质母细胞瘤(GBM)患者中,上调的midkine(MDK)限制了替莫唑胺(TMZ)带来的生存益处。RNA 干扰(RNAi)和 CRISPR-Cas9 基因编辑技术是调节 MDK 表达的有吸引力的方法。然而,将这些生物制剂输送到 GBM 组织具有挑战性。在这里,我们展示了一种聚合物锁定融合脂质体(Plofsome),它可以穿过血脑屏障(BBB),将短干扰 RNA 或 CRISPR-Cas9 核糖核蛋白复合物输送到 GBM 细胞的细胞质中。Plofsome的设计原理是利用无踪活性氧(ROS)可清除连接体将 "锁 "整合到融合脂质体中,这样只有在穿过血脑屏障并进入高ROS水平的GBM组织后才会发生融合。我们的研究结果表明,Plofsomes抑制MDK可显著降低TMZ耐药性,并抑制正位脑肿瘤模型中GBM的生长。重要的是,Plofsomes只对肿瘤部位有效,对正常组织无效,这提高了RNAi和CRISPR-Cas9联合疗法的安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
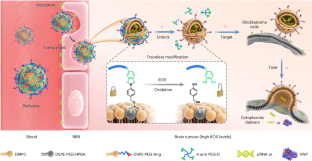

Polymer-locking fusogenic liposomes for glioblastoma-targeted siRNA delivery and CRISPR–Cas gene editing
In patients with glioblastoma (GBM), upregulated midkine (MDK) limits the survival benefits conferred by temozolomide (TMZ). RNA interference (RNAi) and CRISPR–Cas9 gene editing technology are attractive approaches for regulating MDK expression. However, delivering these biologics to GBM tissue is challenging. Here we demonstrate a polymer-locking fusogenic liposome (Plofsome) that can be transported across the blood–brain barrier (BBB) and deliver short interfering RNA or CRISPR–Cas9 ribonucleoprotein complexes into the cytoplasm of GBM cells. Plofsome is designed by integrating a ‘lock’ into the fusogenic liposome using a traceless reactive oxygen species (ROS)-cleavable linker so that fusion occurs only after crossing the BBB and entering the GBM tissue with high ROS levels. Our results showed that MDK suppression by Plofsomes significantly reduced TMZ resistance and inhibited GBM growth in orthotopic brain tumour models. Importantly, Plofsomes are effective only at tumour sites and not in normal tissues, which improves the safety of combined RNAi and CRISPR–Cas9 therapeutics. Delivering gene editing materials to the brain for glioblastoma therapy can boost the efficacy of chemotherapy. Here the authors reduce resistance to temozolomide using a reactive oxygen species-sensitive polymer-locking fusogenic liposome that can cross the blood–brain barrier and deliver short interfering RNA or CRISPR–Cas to glioblastoma with high specificity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


