核蛋白酶体在自噬妥协过程中缓冲细胞质蛋白质
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
自噬是一种保守的途径,细胞质内容物被自噬体吞噬,然后与溶酶体融合使其降解。自噬核心基因突变会导致神经系统疾病,帕金森病和亨廷顿病等神经退行性疾病也会出现自噬缺陷。因此,我们试图利用酵母筛选获得的数据,寻找自噬基因缺失的人类细胞的负遗传相互作用,如合成致死率,从而了解自噬基因缺失的细胞容易受到的细胞通路干扰。这些数据显示,蛋白酶体和核孔复合体成分的缺失会导致自噬无效细胞中类似于合成适存性丧失的协同活力变化。这可归因于自噬缺乏时蛋白质从细胞质到细胞核的转运,以及随后细胞核蛋白酶体对这些昔日细胞质蛋白质的降解。由于亨廷顿氏病的自噬和细胞质到细胞核的转运都存在缺陷,因此这类细胞更容易受到这些合成相互作用导致的蛋白稳态紊乱的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
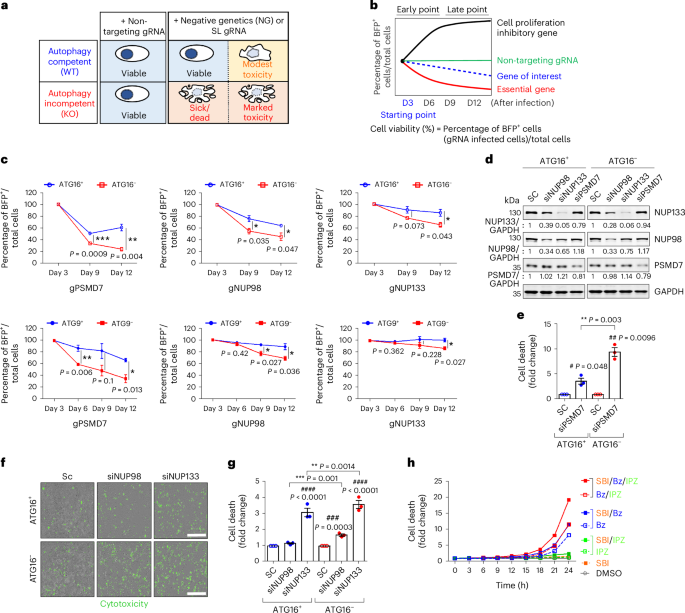
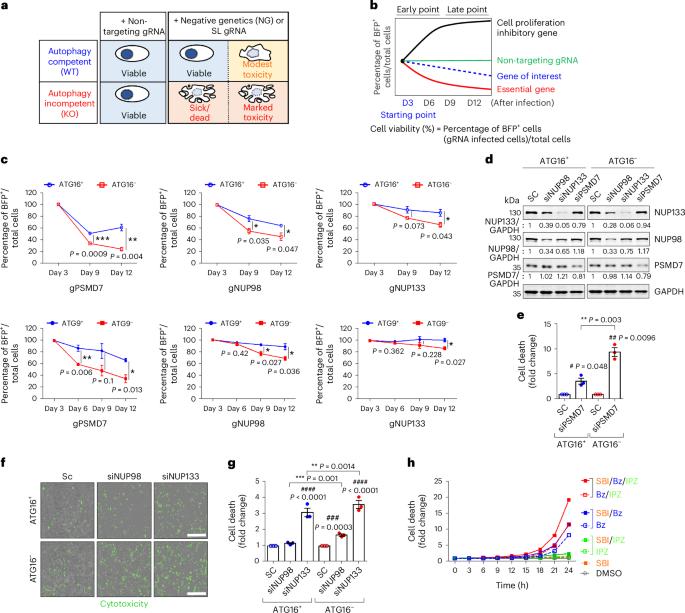
Nuclear proteasomes buffer cytoplasmic proteins during autophagy compromise
Autophagy is a conserved pathway where cytoplasmic contents are engulfed by autophagosomes, which then fuse with lysosomes enabling their degradation. Mutations in core autophagy genes cause neurological conditions, and autophagy defects are seen in neurodegenerative diseases such as Parkinson’s disease and Huntington’s disease. Thus, we have sought to understand the cellular pathway perturbations that autophagy-perturbed cells are vulnerable to by seeking negative genetic interactions such as synthetic lethality in autophagy-null human cells using available data from yeast screens. These revealed that loss of proteasome and nuclear pore complex components cause synergistic viability changes akin to synthetic fitness loss in autophagy-null cells. This can be attributed to the cytoplasm-to-nuclear transport of proteins during autophagy deficiency and subsequent degradation of these erstwhile cytoplasmic proteins by nuclear proteasomes. As both autophagy and cytoplasm-to-nuclear transport are defective in Huntington’s disease, such cells are more vulnerable to perturbations of proteostasis due to these synthetic interactions. Park et al. show that cells with impaired autophagy shuttle cytoplasmic proteins to the nucleus for degradation by nuclear proteasomes, revealing synergistic vulnerabilities in diseases where autophagy and nucleocytoplasmic transport are compromised.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


