了解电催化 C(sp3)-C(sp3)碎片化和加氧反应之间的相互作用
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
实现 C(sp3)-C(sp3)和 C(sp3)-H 键的选择性电催化活化,是实现电力驱动的化学品合成、塑料的可持续升级以及开发使用高能量液体燃料的燃料电池的关键。当碳氢化合物暴露于氧化偏压下的电极时,会发生 C-C 键断裂和氧合反应。目前,我们对这些途径的分叉缺乏控制。在此,我们对烷基转化反应的复杂网络进行了深入研究,结果表明在氧化电位下,吸附的丁烷会转化为吸附的 CHx 片段,在氧化为吸附的 CO 之前,CHx 片段会被解吸为甲烷。确定了 C-C 断裂和氧合之间的支点后,我们就可以根据特定吸附物的解吸电位和中间氧化的动力学原理,通过应用脉冲电位来控制选择性。我们的发现为改进燃料电池催化剂提供了设计标准,并为电合成途径中选择性 C-C 裂解打开了大门。本文章由计算机程序翻译,如有差异,请以英文原文为准。
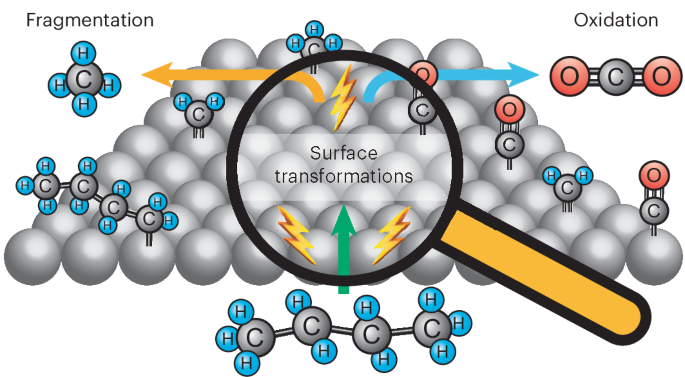
Understanding the interplay between electrocatalytic C(sp3)‒C(sp3) fragmentation and oxygenation reactions
Achieving the selective electrocatalytic activation of C(sp3)–C(sp3) and C(sp3)−H bonds is key to enabling the electricity-driven synthesis of chemicals, the sustainable upgrading of plastics and the development of fuel cells operating on energy-dense liquid fuels. When exposed to electrodes under oxidative bias, hydrocarbons undergo both C–C bond fragmentation and oxygenation. Currently, we lack control over the bifurcation of these pathways. Here we provide insights into the complex network of alkyl transformation reactions, showing that under oxidizing potentials, adsorbed butane transforms to adsorbed CHx fragments, which can be desorbed as methane before oxidation to adsorbed CO. Identifying the branchpoint between C‒C fragmentation and oxygenation allowed us to steer selectivity by applying pulsed potentials tailored to the desorption potential of specific adsorbates and the kinetics of intermediate oxidation. Our findings provide design criteria for improved fuel cell catalysts and open the door to selective C‒C cleavage in electrosynthetic pathways. The electrochemical activation of alkanes on metal catalysts is a complex process that is not fully understood. Now an electrochemical protocol is put forward to isolate the adsorption, fragmentation and oxygenation potential-dependent steps of butane activation on a platinum electrode and derive its intricate reaction network.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


