巢状神经回路的活动驱动果蝇发出不同的求偶歌曲
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
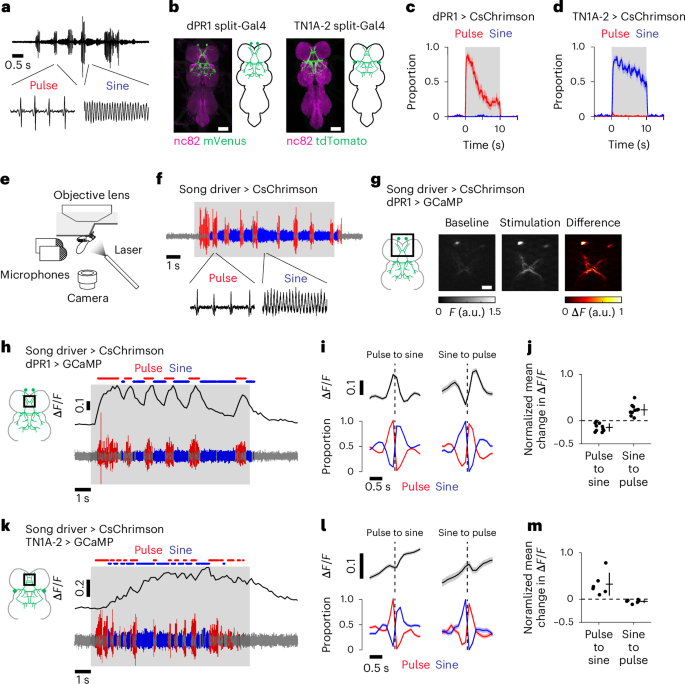
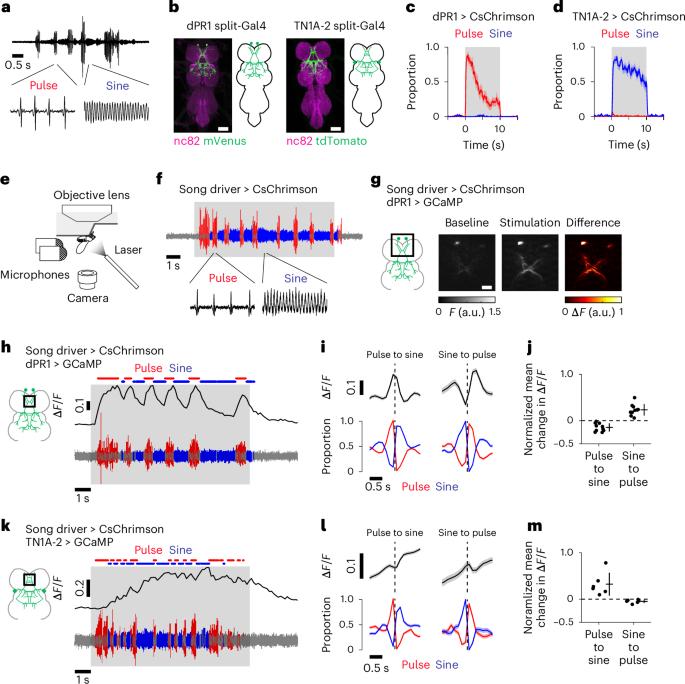
运动系统通过执行不同的运动程序将行为序列模式化,但不同的运动动作是如何在每一时刻被控制的仍不清楚。在这里,我们研究了果蝇控制不同求爱歌曲的神经回路机制。求偶的雄性果蝇会在脉冲和正弦两种类型的歌声之间快速交替。通过记录求偶蝇腹侧神经索中的钙信号,我们发现一个神经群在两种歌声中都处于活跃状态,而一个扩大的神经群(包括第一个神经群中的神经元)在脉冲歌声中处于活跃状态。大脑记录显示,这种嵌套激活模式存在于歌唱所需的两条下降通路中。连接组学分析表明,这两条下行通路为腹侧神经索神经元提供结构化输入,其方式与其激活模式一致。这些结果表明,嵌套的前运动回路活动由不同的下降信号引导,能够在运动动作之间快速切换。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Activity of nested neural circuits drives different courtship songs in Drosophila
Motor systems implement diverse motor programs to pattern behavioral sequences, yet how different motor actions are controlled on a moment-by-moment basis remains unclear. Here, we investigated the neural circuit mechanisms underlying the control of distinct courtship songs in Drosophila. Courting males rapidly alternate between two types of song: pulse and sine. By recording calcium signals in the ventral nerve cord in singing flies, we found that one neural population is active during both songs, whereas an expanded neural population, which includes neurons from the first population, is active during pulse song. Brain recordings showed that this nested activation pattern is present in two descending pathways required for singing. Connectomic analysis reveals that these two descending pathways provide structured input to ventral nerve cord neurons in a manner consistent with their activation patterns. These results suggest that nested premotor circuit activity, directed by distinct descending signals, enables rapid switching between motor actions. Activation of nested, but not discrete, neural circuits drives alternative courtship songs in male Drosophila melanogaster, providing further insight into how the nervous system can drive the same motor systems to rapidly switch between different actions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


