揭示金属氧化物脱羧芳基化反应的立体选择性
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
目前,脱羧交叉偶联方法已广泛应用于药物发现研究,为战略性逆合成分析和基于自由基的键断开奠定了基础。在此,我们揭示了取代环羧酸的金属氧化物脱羧芳基化的非对映选择性。通过对条件、配体和添加剂的审慎筛选,光氧化促进的镍催化脱羧芳基化反应具有很高的非对映选择性,从而可以模块化地获得复杂的环状结构。该反应可容忍各种官能团,包括游离醇和碱性胺。对 1,2-、1,3- 和 1,4- 甲基环己烷的 L2ArXNi(III)R 复合物进行的计算 DFT 结构和能量研究表明,所有赤道构象都优于赤道/轴构象。根据计算的自由能和产物异构体分布,L2ArXNi(III)R 会发生还原消除,是非对称的决定性中间体。为了展示这种方法在药物发现和工艺开发方面的稳健性和可扩展性,成功地进行了一次 200 毫摩尔(51.5 克)的流动反应。最后,为了展示这种偶联方法的简化能力,我们利用它将最近获得 FDA 批准用于治疗成人阵发性夜间血红蛋白尿(PNH)疾病的药物 Iptacopan (LNP023) 的合成从之前报道的 12 步(外消旋路线)缩短为 4 步(对映体选择路线)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
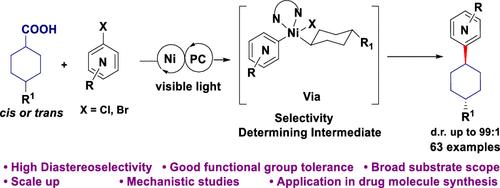
Unveiling the Stereoselectivity Aspects of Metallaphotoredox Decarboxylative Arylation
Decarboxylative cross-coupling methodologies are now widely employed in pharmaceutical drug discovery research, forming the basis of strategic retrosynthetic analysis and radical-based bond disconnections. Herein, we unveil the diastereoselectivity aspects of metallaphotoredox decarboxylative arylation of substituted cyclic carboxylic acids. Through judicious screening of conditions, ligands, and additives, photoredox-promoted Ni-catalyzed decarboxylative arylation was rendered highly diastereoselective, enabling modular access to complex cyclic architectures. The reaction tolerates various functional groups, including free alcohols and basic amines. Computational DFT structural and energetic studies of L2ArXNi(III)R complexes of 1,2-, 1,3-, and 1,4-methyl cyclohexanes revealed a conformational preference for all equatorial over equatorial/axial conformations. The L2ArXNi(III)R, which undergoes reductive elimination, is the diastereo-determining intermediate based on calculated free energies and product isomeric distribution. To showcase the robustness and scalability of this methodology for drug discovery and process development, a 200 mmol (51.5 g) reaction was successfully carried out in flow. Finally, to demonstrate the simplifying power of this coupling approach, we employed it to truncate the synthesis of Iptacopan (LNP023), a recently FDA-approved drug for treating Paroxysmal nocturnal hemoglobinuria (PNH) disorder in adults, from the previously reported 12-steps (racemic route) to 4-steps (enantioselective route).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


