产生气候活性气体二甲基硫醚的 S-甲基转移酶广泛存在于各种海洋细菌中
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
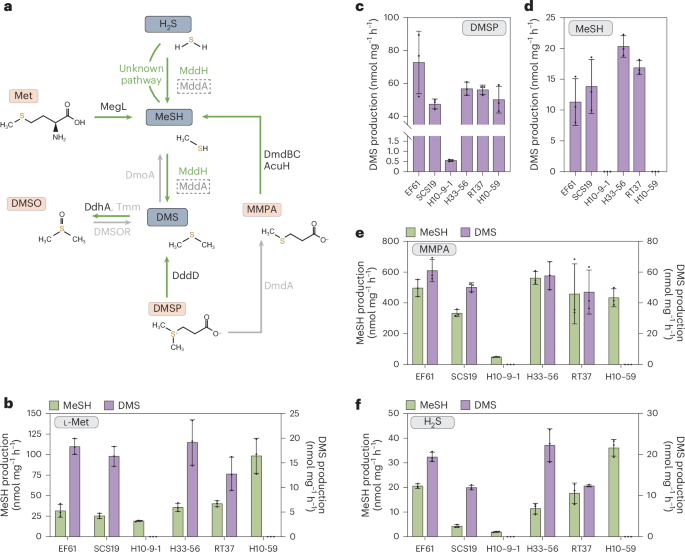
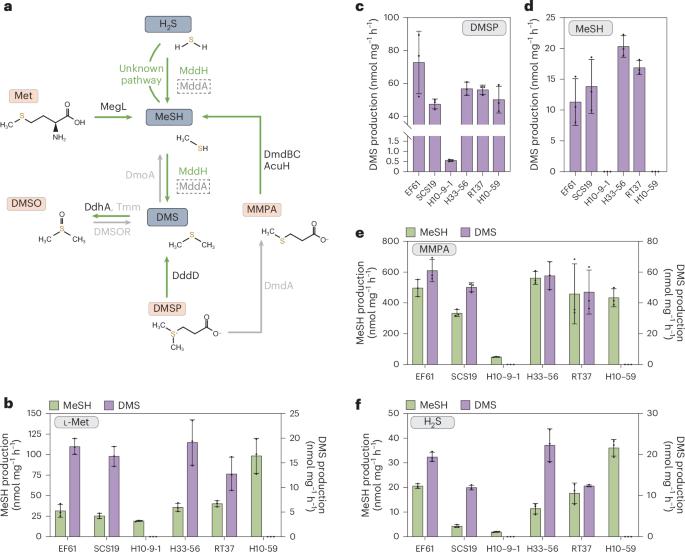
硫化氢(H2S)、甲硫醇(MeSH)和二甲基硫醚(DMS)是丰富的硫化气体,在生物地球化学循环、趋化作用和/或气候调节中发挥作用。海洋渗透溶质二甲基硫代丙酸酯(DMSP)的分解代谢是 DMS 和 MeSH 的主要来源,但这两种物质也是通过 MddA(一种 H2S 和 MeSH S-甲基转移酶,其基因在土壤中含量丰富,但在海洋环境中却很少见)对 H2S 进行 S-甲基转移的结果。在这里,我们发现了依赖于 S-腺苷蛋氨酸(SAM)的 MeSH 和 H2S S-甲基转移酶 "MddH",它广泛存在于多种海洋细菌以及一些淡水和土壤细菌中。据预测,mddH 在海水和沿海沉积物细菌中的含量分别高达 ~5% 和 ~15%,大大高于 mddA。此外,海洋 mddH 的转录水平与最丰富的 DMSP 裂解酶基因 dddP 相似。这项研究表明,H2S 和 MeSH S-甲基化途径在海洋环境中的重要性被严重低估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An S-methyltransferase that produces the climate-active gas dimethylsulfide is widespread across diverse marine bacteria
Hydrogen sulfide (H2S), methanethiol (MeSH) and dimethylsulfide (DMS) are abundant sulfur gases with roles in biogeochemical cycling, chemotaxis and/or climate regulation. Catabolism of the marine osmolyte dimethylsulfoniopropionate (DMSP) is a major source of DMS and MeSH, but both also result from S-methylation of H2S via MddA, an H2S and MeSH S-methyltransferase whose gene is abundant in soil but scarce in marine environments. Here we identify the S-adenosine methionine (SAM)-dependent MeSH and H2S S-methyltransferase ‘MddH’, which is widespread in diverse marine bacteria and some freshwater and soil bacteria. mddH is predicted in up to ~5% and ~15% of seawater and coastal sediment bacteria, respectively, which is considerably higher than mddA. Furthermore, marine mddH transcript levels are similar to those for the most abundant DMSP lyase gene dddP. This study implies that the importance of H2S and MeSH S-methylation pathways in marine environments is significantly underestimated. The S-methyltransferase enzyme MddH produces DMS from hydrogen sulfide and methanethiol and its gene abundance rivals that of other known genes whose products generate DMS in marine environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


