基于热重分析和 DFT 模拟的过氧化苯甲酰热分解动力学
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
过氧化苯甲酰的热分解特性是指导过氧化苯甲酰安全应用的重要依据。通过多重扫描速率热重分析和密度泛函理论(DFT)计算,研究了过氧化苯甲酰热分解过程的特征温度和发热特性。利用等效转化法计算了过氧化苯甲酰热分解反应的动力学参数,并通过 Coats-Redfern 法和主图法探讨了过氧化苯甲酰热分解反应的最可能机理函数。结果表明,过氧化苯甲酰热分解反应遵循随机成核和核生长的反应动力学机制,但在不同反应阶段遵循不同的模型。此外,过氧化苯甲酰的热分解是一个复杂的过程,可能涉及多个平行竞争反应。这些结果有利于过氧化苯甲酰在工业生产中的安全应用和防止损失。本文章由计算机程序翻译,如有差异,请以英文原文为准。
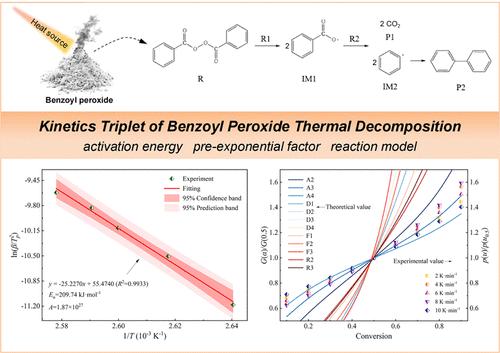
Thermal Decomposition Kinetics of Benzoyl Peroxide Based on Thermogravimetric Analysis and DFT Simulations
The thermal decomposition characteristic of benzoyl peroxide is a significant basis for guiding the safe application of benzoyl peroxide. The characteristic temperature and heat generation characteristics of the benzoyl peroxide thermal decomposition process were studied through multiple scan rate thermogravimetric analyses and density functional theory (DFT) calculations. The kinetic parameters of the benzoyl peroxide thermal decomposition reaction were calculated by using the isoconversional methods, and the most probable mechanism function of the benzoyl peroxide thermal decomposition reaction was explored via the Coats–Redfern method and the master-plots method. The results showed that the thermal decomposition reaction of benzoyl peroxide followed the reaction kinetics mechanism of random nucleation and nuclei growth, but it followed different models at the different reaction phases. Furthermore, the thermal decomposition of benzoyl peroxide is a complex process that may involve multiple parallel competitive reactions. The results benefit the safe application and loss prevention of benzoyl peroxide in industrial production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


