利用 DPA-Zn 改性纳米颗粒将 CRISPR 系统靶向送入肿瘤,编辑谷氨酰胺代谢,从而治疗癌症
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
肿瘤细胞为维持增殖和存活进行着独特的新陈代谢,因此针对新陈代谢途径进行治疗是一种很有吸引力的肿瘤治疗方法。谷氨酰胺代谢在促进肿瘤生长和调节肿瘤微环境方面发挥着至关重要的作用。然而,谷氨酰胺代谢靶向疗法的临床转化面临着效率低下和全身毒性效应的问题。在这里,我们构建了具有生物相容性和功能性的聚合物纳米粒子,将CRISPR-Cas9送入肿瘤,实现高效、同步的基因编辑,从而切断表达谷氨酰胺酶(GLS1)和磷酸核糖基焦磷酸酰胺转移酶(PPAT)的两个基因,操纵谷氨酰胺代谢。结果表明,对谷氨酰胺代谢的遗传操作能显著抑制肿瘤的发展和转移,同时还能有利地改变肿瘤的微环境。重要的是,这种方法提高了抗肿瘤免疫力,促进了长期免疫记忆。这项工作凸显了通过基因编辑同时靶向多种谷氨酰胺代谢途径的潜力,为癌症治疗提供了一种前景广阔的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
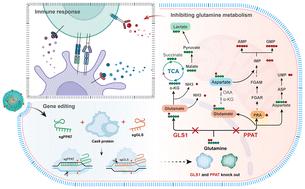
Targeting delivery of CRISPR systems into tumours to edit glutamine metabolism for cancer therapy by DPA-Zn-modified nanoparticles†
Tumour cells exhibit distinct metabolism to sustain their proliferation and survival, making targeting metabolic pathways an appealing option for tumour therapy. Glutamine metabolism plays a crucial role in fuelling tumour growth and modulating the tumour microenvironment. However, the clinical translation of glutamine metabolism-targeting therapies faces poor efficiency and systemic toxic effects. Here, we constructed biocompatible and functional polymer nanoparticles to deliver CRISPR-Cas9 into tumours for efficient and simultaneous gene editing, which can cut off two genes that express glutaminase (GLS1) and phosphoribosyl pyrophosphate amidotransferase (PPAT) to manipulate glutamine metabolism. The results demonstrated that genetic manipulation of glutamine metabolism significantly inhibited tumour development and metastasis while also favourably altering the tumour microenvironment. Importantly, this method improved antitumour immunity and promoted long-term immunological memory. This work highlights the potential of simultaneously targeting multiple glutamine metabolic pathways through gene editing, providing a promising strategy for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


