质粒编码的插入序列促进临床肠杆菌的快速适应
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
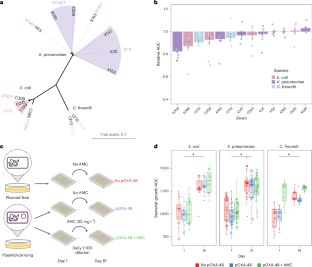
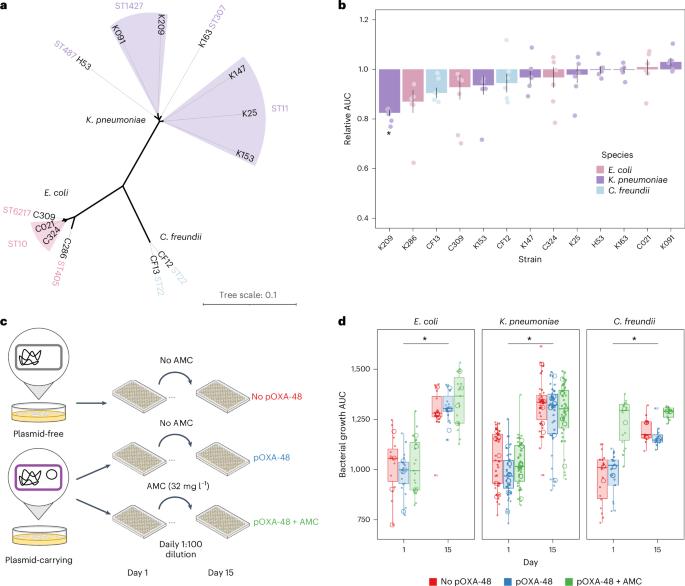
质粒是细菌中常见的染色体外遗传元件。众所周知,质粒通过水平基因转移促进细菌进化,最近的分析表明,质粒还能促进基因组内的适应。然而,除了水平基因转移之外,质粒作为细菌进化催化剂的作用还鲜有研究。在本研究中,我们研究了一种广泛存在的共轭质粒 pOXA-48 对几种耐多药临床肠杆菌进化的影响。结合实验和患者内部进化分析,我们发现质粒 pOXA-48 通过质粒编码的插入序列 1(IS1)元件的转座促进了细菌的进化。具体来说,IS1 介导的基因失活加快了临床菌株在体外的适应速度,并促进了患者在肠道微生物群中的适应。我们破译了质粒介导的 IS1 转座激增的内在机制,揭示了由 IS1 基因组拷贝数调节的负反馈回路。鉴于 IS 元素在细菌质粒中所占比例过高,我们的研究结果表明,质粒介导的 IS1 转座是细菌迅速适应的关键机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Plasmid-encoded insertion sequences promote rapid adaptation in clinical enterobacteria
Plasmids are extrachromosomal genetic elements commonly found in bacteria. They are known to fuel bacterial evolution through horizontal gene transfer, and recent analyses indicate that they can also promote intragenomic adaptations. However, the role of plasmids as catalysts of bacterial evolution beyond horizontal gene transfer is poorly explored. In this study, we investigated the impact of a widespread conjugative plasmid, pOXA-48, on the evolution of several multidrug-resistant clinical enterobacteria. Combining experimental and within-patient evolution analyses, we unveiled that plasmid pOXA-48 promotes bacterial evolution through the transposition of plasmid-encoded insertion sequence 1 (IS1) elements. Specifically, IS1-mediated gene inactivation expedites the adaptation rate of clinical strains in vitro and fosters within-patient adaptation in the gut microbiota. We deciphered the mechanism underlying the plasmid-mediated surge in IS1 transposition, revealing a negative feedback loop regulated by the genomic copy number of IS1. Given the overrepresentation of IS elements in bacterial plasmids, our findings suggest that plasmid-mediated IS1 transposition represents a crucial mechanism for swift bacterial adaptation. Combining experimental and within-patient evolution analyses, the authors show that the widespread conjugative plasmid pOXA-48 promotes bacterial evolution through the transposition of plasmid-encoded insertion sequence IS1 elements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


