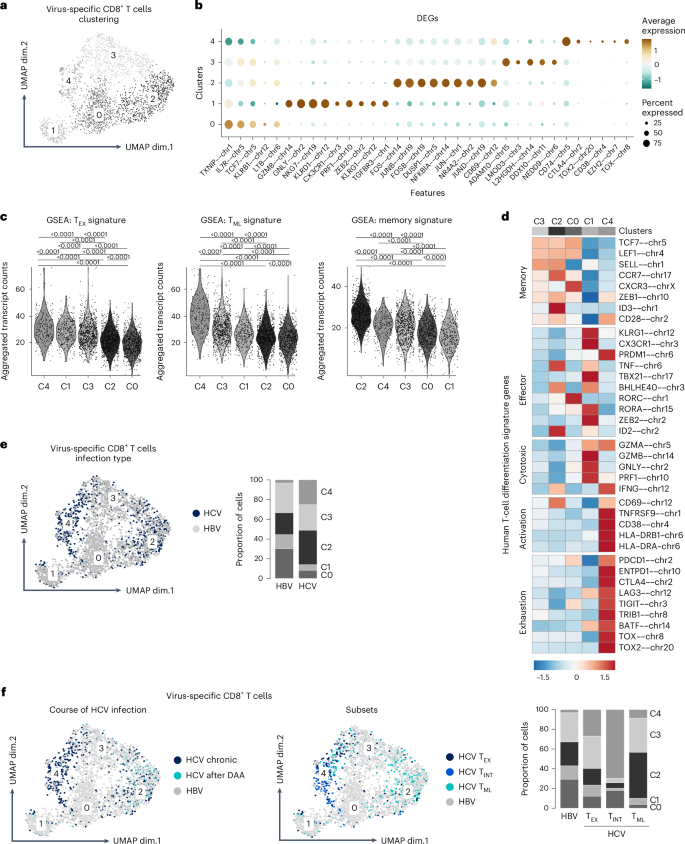
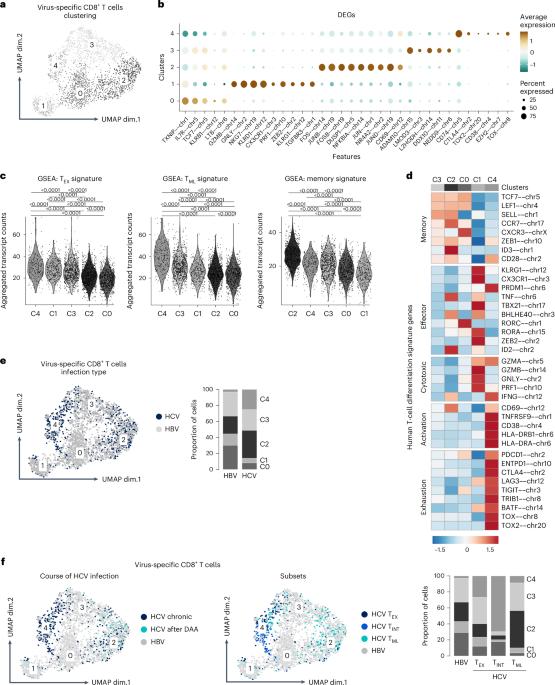
减弱的效应 T 细胞与控制慢性 HBV 感染有关
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
乙型肝炎病毒(HBV)特异性 CD8+ T 细胞在急性缓解型 HBV 感染期间发挥着主导作用,但在人类慢性 HBV 感染期间功能受损。这些功能缺陷与代谢和表型异质性有关,但目前仍不清楚不同的 HBV 特异性 CD8+ T 细胞亚群在多大程度上仍能抑制病毒复制。我们通过在慢性 HBV 感染的不同阶段对 HBV 特异性 CD8+ T 细胞进行深度剖析、功能测试和扰乱来解决这个问题。我们的数据揭示了一种效应 CD8+ T 细胞衰减机制,它与传统的 CD8+ T 细胞衰竭同时出现。衰减的 HBV 特异性 CD8+ T 细胞具有细胞毒性特性,其效应分化程序受到抑制,由抗原识别和 TGFβ 信号转导决定,并与慢性 HBV 感染期间的病毒控制有关。这些观察结果确定了在人类慢性病毒感染背景下与免疫效力相关的CD8+T细胞独特亚群,具有免疫治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Attenuated effector T cells are linked to control of chronic HBV infection
Hepatitis B virus (HBV)-specific CD8+ T cells play a dominant role during acute-resolving HBV infection but are functionally impaired during chronic HBV infection in humans. These functional deficits have been linked with metabolic and phenotypic heterogeneity, but it has remained unclear to what extent different subsets of HBV-specific CD8+ T cells still suppress viral replication. We addressed this issue by deep profiling, functional testing and perturbation of HBV-specific CD8+ T cells during different phases of chronic HBV infection. Our data revealed a mechanism of effector CD8+ T cell attenuation that emerges alongside classical CD8+ T cell exhaustion. Attenuated HBV-specific CD8+ T cells were characterized by cytotoxic properties and a dampened effector differentiation program, determined by antigen recognition and TGFβ signaling, and were associated with viral control during chronic HBV infection. These observations identify a distinct subset of CD8+ T cells linked with immune efficacy in the context of a chronic human viral infection with immunotherapeutic potential. Heim et al. investigate the role of CD8+ T cells specific to HBV polymerase in the context of chronic HBV infection. They identify a unique subset of CD8+ T cells with an attenuated effector function. The attenuation is driven by TGFβ signaling, offering new insights into the immune landscape of chronic HBV infection and suggesting potential therapeutic avenues for modulating these cells to enhance viral control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


