对映选择性 Csp3-Csp3 交叉偶联的辐射控制
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在复杂分子的合成过程中,Csp3-Csp3 键的对映选择性形成仍然是一个巨大的挑战。现在,我们已经开发出一种光催化系统,用于α-氨基 Csp3-H 键的对映选择性烷基化,该系统可促进生成两种不同的烷基自由基,然后在手性镍中心进行交叉偶联。本文章由计算机程序翻译,如有差异,请以英文原文为准。
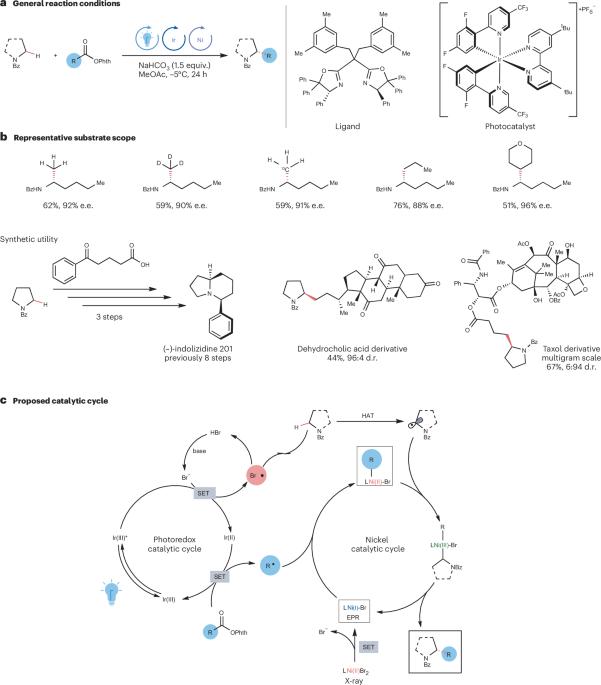
Radical control for enantioselective Csp3–Csp3 cross-coupling
The enantioselective formation of Csp3–Csp3 bonds is still a substantial challenge in the synthesis of complex molecules. Now, a photocatalytic system has been developed for the enantioselective alkylation of α-amino Csp3−H bonds that promotes the generation of two different alkyl radicals, followed by their cross-coupling at a chiral nickel centre.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


