α和β放射性核素对转移性前列腺癌的免疫效应
IF 12.1
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
体外放射治疗用于器官封闭性前列腺癌的根治性治疗和转移性疾病的病灶治疗,而使用标记的前列腺特异性膜抗原配体和镭-223(223Ra)的分子放射治疗则适用于转移性前列腺癌,与常规治疗相比,该疗法在症状控制和总生存率方面有显著改善。前列腺癌被认为是一种免疫功能低下的肿瘤,因此有关治疗对免疫反应影响的研究十分有限。不过,新出现的数据支持放疗诱导前列腺癌免疫反应的观点,但这种反应是抗肿瘤反应还是促肿瘤反应取决于放疗方案,也取决于细胞系。体外数据表明,单剂量放疗方案比分次放疗方案诱导的免疫抑制作用更大;目前对分子放疗药物诱导的免疫反应了解较少,但有证据表明,这些药物可能会诱导免疫抑制性全身免疫反应,表现为抑制性检查点分子(如程序性细胞死亡 1 配体 1 和 2)的表达增加,这些变化可能与临床反应有关。不同的放疗模式可诱导不同的免疫特征,从而激活或抑制免疫介导的肿瘤杀伤作用,而目前用于前列腺癌研究的临床前模型还不是研究放疗诱导免疫反应复杂性的最佳模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
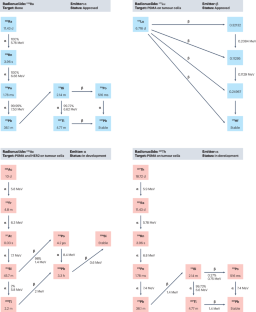
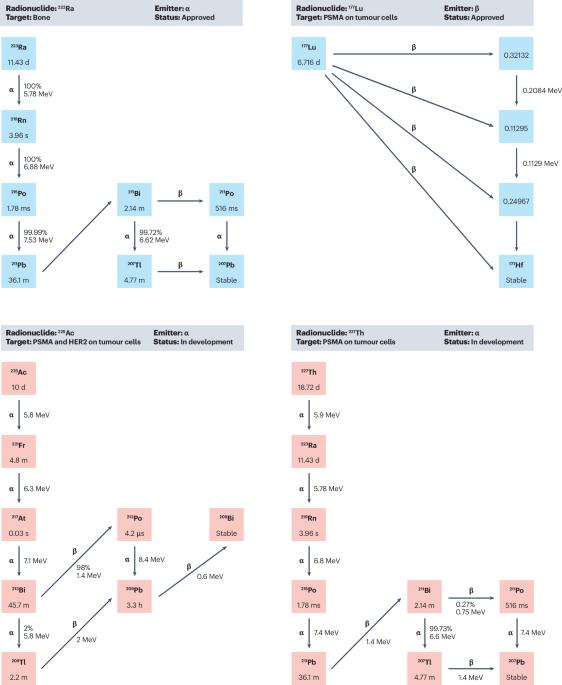
Immune effects of α and β radionuclides in metastatic prostate cancer
External beam radiotherapy is used for radical treatment of organ-confined prostate cancer and to treat lesions in metastatic disease whereas molecular radiotherapy with labelled prostate-specific membrane antigen ligands and radium-223 (223Ra) is indicated for metastatic prostate cancer and has demonstrated substantial improvements in symptom control and overall survival compared with standard-of-care treatment. Prostate cancer is considered an immunologically cold tumour, so limited studies investigating the treatment-induced effects on the immune response have been completed. However, emerging data support the idea that radiotherapy induces an immune response in prostate cancer, but whether the response is an antitumour or pro-tumour response is dependent on the radiotherapy regime and is also cell-line dependent. In vitro data demonstrate that single-dose radiotherapy regimes induce a greater immune-suppressive profile than fractionated regimes; less is known about the immune response induced by molecular radiotherapy agents, but evidence suggests that these agents might induce an immune-suppressive systemic immune response, indicated by increased expression of inhibitory checkpoint molecules such as programmed cell death 1 ligand 1 and 2, and that these changes could be associated with clinical response. Different radiotherapy modalities can induce distinct immune profiles, which can either activate or suppress immune-mediated tumour killing and the current preclinical models used for prostate cancer research are not yet optimal for studying the complexity of the radiotherapy-induced immune response. This Review focusses on what is known about the interactions between conventional radiotherapy and the immune system. The authors discuss how this knowledge can be applied to investigate gaps regarding the immune interactions of molecular radiotherapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Urology
医学-泌尿学与肾脏学
CiteScore
12.50
自引率
2.60%
发文量
123
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Urology is part of the Nature Reviews portfolio of journals.Nature Reviews' basic, translational and clinical content is written by internationally renowned basic and clinical academics and researchers. This journal targeted readers in the biological and medical sciences, from the postgraduate level upwards, aiming to be accessible to professionals in any biological or medical discipline.
The journal features authoritative In-depth Reviews providing up-to-date information on topics within a field's history and development. Perspectives, News & Views articles, and the Research Highlights section offer topical discussions and opinions, filtering primary research from various medical journals.
Covering a wide range of subjects, including andrology, urologic oncology, and imaging, Nature Reviews provides valuable insights for practitioners, researchers, and academics within urology and related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


