肿瘤突变负荷:临床实用性、挑战和新的改进。
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
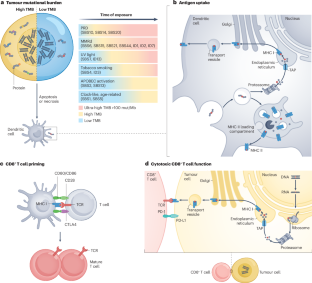
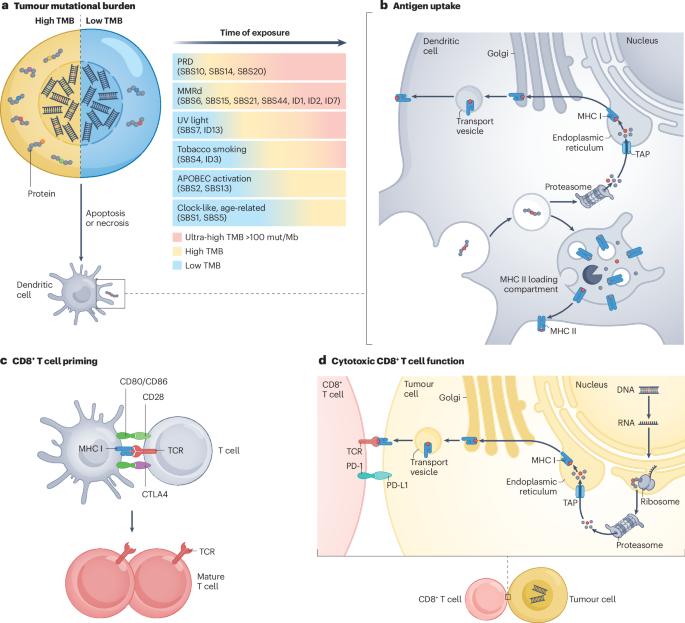
肿瘤突变负荷(TMB)是指癌症基因组中存在的体细胞非同义突变的总数,在不同癌症类型之间和癌症类型内部都存在差异。第一波回顾性和前瞻性研究发现,TMB 是对免疫检查点抑制剂反应的预测性生物标志物,并最终根据 Keynote-158 试验的数据,批准 pembrolizumab 用于 TMB 高的肿瘤患者的疾病诊断。尽管这项试验的结果是否适用于所有癌症类型以及 TMB 的最佳阈值尚待确定,但对 TMB 的研究正沿着三个主要途径推进:通过实验室流程中严格的质量控制措施加强 TMB 评估,包括减少混杂因素,如小组范围有限和肿瘤纯度低;通过纳入创新概念完善传统 TMB 框架,如克隆、持续或 HLA 校正 TMB、肿瘤新抗原负荷和突变特征;以及将 TMB 与 PD-L1 表达、微卫星不稳定性、免疫基因表达谱和肿瘤免疫背景等已有和新兴生物标记物相结合。鉴于 TMB 在癌症发病机制和免疫系统识别肿瘤能力中的关键作用,深刻理解 TMB 的基本原理和持续演化对肿瘤学领域至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumour mutational burden: clinical utility, challenges and emerging improvements
Tumour mutational burden (TMB), defined as the total number of somatic non-synonymous mutations present within the cancer genome, varies across and within cancer types. A first wave of retrospective and prospective research identified TMB as a predictive biomarker of response to immune-checkpoint inhibitors and culminated in the disease-agnostic approval of pembrolizumab for patients with TMB-high tumours based on data from the Keynote-158 trial. Although the applicability of outcomes from this trial to all cancer types and the optimal thresholds for TMB are yet to be ascertained, research into TMB is advancing along three principal avenues: enhancement of TMB assessments through rigorous quality control measures within the laboratory process, including the mitigation of confounding factors such as limited panel scope and low tumour purity; refinement of the traditional TMB framework through the incorporation of innovative concepts such as clonal, persistent or HLA-corrected TMB, tumour neoantigen load and mutational signatures; and integration of TMB with established and emerging biomarkers such as PD-L1 expression, microsatellite instability, immune gene expression profiles and the tumour immune contexture. Given its pivotal functions in both the pathogenesis of cancer and the ability of the immune system to recognize tumours, a profound comprehension of the foundational principles and the continued evolution of TMB are of paramount relevance for the field of oncology. Tumour mutational burden (TMB), reflecting the number of mutations present in the DNA of a tumour, is a biologically appealing biomarker of a response to immune-checkpoint inhibitors (ICIs). Nonetheless, the clinical predictive value of TMB for ICI response has thus far been mixed, with robust associations seen only for a few ICI-responsive cancer types. In this Review, the authors summarize the available data on TMB and discuss ongoing research efforts to improve the clinical utility of this biomarker.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


