透明质酸在肾脏发育、生理和疾病中的作用。
IF 28.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
组织微环境中的透明质酸(HA)基质通过调节炎症信号、内皮-间质转化和细胞迁移,对维持体内平衡至关重要。在发育过程中,HA 的共价修饰和渗透膨胀会产生机械力,从而启动中肠旋转、血管模式化和分支形态发生。HA 与其主要的细胞表面受体 CD44 一起,在细胞表面建立了一个物理化学支架,促进正常生理所需的生长因子和受体的相互作用和聚集。高分子量 HA、肿瘤坏死因子刺激基因 6、Pentraxin 3 和 CD44 可形成稳定的细胞外基质,促进组织再生并减少炎症。相反,高分子量 HA 被透明质酸酶分解成解聚片段,会引发炎症信号、白细胞迁移和血管生成,导致肾脏疾病中的组织损伤和纤维化。由于透明质酸酶的动态调节和组织特异性功能,针对透明质酸酶代谢的研究具有挑战性。然而,通过靶向 HA 的结合伙伴来调节 HA 基质的功能,有望成为恢复组织稳态和减轻病理过程的一种治疗策略。有必要在这一领域开展进一步的研究,以便针对肾脏和其他以 HA 代谢失调为特征的疾病开发新的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
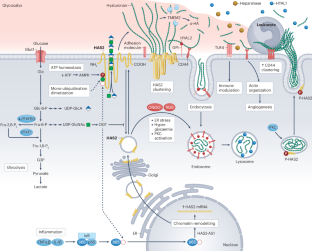
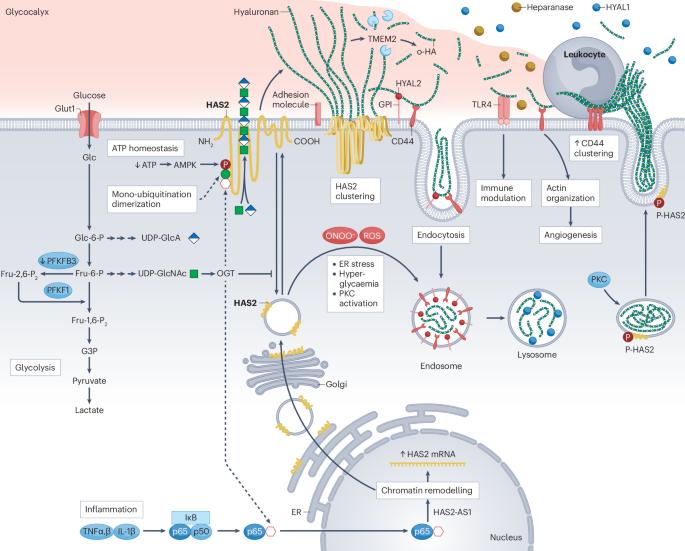
The roles of hyaluronan in kidney development, physiology and disease
The hyaluronan (HA) matrix in the tissue microenvironment is crucial for maintaining homeostasis by regulating inflammatory signalling, endothelial–mesenchymal transition and cell migration. During development, covalent modifications and osmotic swelling of HA create mechanical forces that initiate midgut rotation, vascular patterning and branching morphogenesis. Together with its main cell surface receptor, CD44, HA establishes a physicochemical scaffold at the cell surface that facilitates the interaction and clustering of growth factors and receptors that is required for normal physiology. High-molecular-weight HA, tumour necrosis factor-stimulated gene 6, pentraxin 3 and CD44 form a stable pericellular matrix that promotes tissue regeneration and reduces inflammation. By contrast, breakdown of high-molecular-weight HA into depolymerized fragments by hyaluronidases triggers inflammatory signalling, leukocyte migration and angiogenesis, contributing to tissue damage and fibrosis in kidney disease. Targeting HA metabolism is challenging owing to its dynamic regulation and tissue-specific functions. Nonetheless, modulating HA matrix functions by targeting its binding partners holds promise as a therapeutic strategy for restoring tissue homeostasis and mitigating pathological processes. Further research in this area is warranted to enable the development of novel therapeutic approaches for kidney and other diseases characterized by dysregulated HA metabolism. Hyaluronan is a critical component of the extracellular matrix, with key roles in tissue homeostasis, cellular signalling and immune responses. Here, the authors describe the roles of hyaluronan in kidney development, adult kidney physiology and kidney disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


