利用 MP3-seq 测序技术大规模并行测量蛋白质与蛋白质之间的相互作用。
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
蛋白质-蛋白质相互作用(PPIs)调控着许多细胞过程,工程化的 PPIs 可用于细胞和基因治疗。在这里,我们介绍了通过测序进行大规模并行 PPI 测量(MP3-seq),这是一种易于使用且高度可扩展的测量 PPI 的酵母双杂交方法。在 MP3-seq 中,DNA 条形码与特定蛋白质对相关联,条形码富集可通过测序读取,从而直接测量相互作用强度。我们的研究表明,MP3-seq 具有高度定量性,可扩展到 100,000 种以上的相互作用。我们将 MP3-seq 应用于描述合理设计的异源二聚体家族之间的相互作用,并研究赋予线圈相互作用特异性的元素。最后,我们使用 AlphaFold-Multimer(AF-M)预测了盘绕异二聚体结构,并训练了基于物理学能量项的线性模型来预测 MP3-seq 值。我们发现,基于 AF-M 的模型对预选相互作用很有价值,但要对相互作用的强度进行定量排序,仍然需要对相互作用进行实验测量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
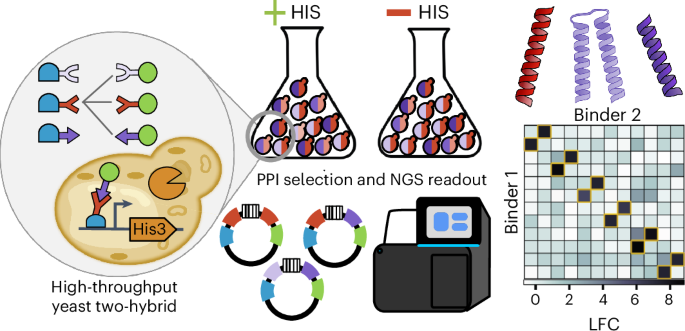
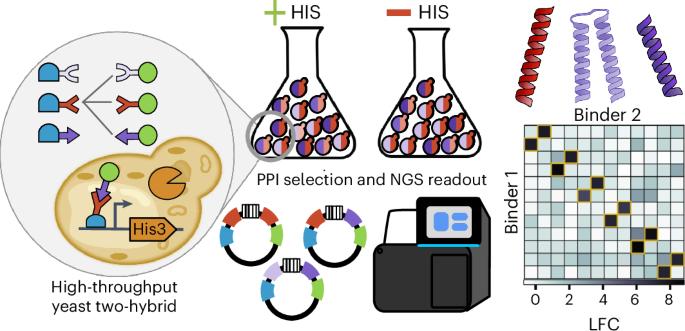
Massively parallel measurement of protein–protein interactions by sequencing using MP3-seq
Protein–protein interactions (PPIs) regulate many cellular processes and engineered PPIs have cell and gene therapy applications. Here, we introduce massively parallel PPI measurement by sequencing (MP3-seq), an easy-to-use and highly scalable yeast two-hybrid approach for measuring PPIs. In MP3-seq, DNA barcodes are associated with specific protein pairs and barcode enrichment can be read by sequencing to provide a direct measure of interaction strength. We show that MP3-seq is highly quantitative and scales to over 100,000 interactions. We apply MP3-seq to characterize interactions between families of rationally designed heterodimers and to investigate elements conferring specificity to coiled-coil interactions. Lastly, we predict coiled heterodimer structures using AlphaFold-Multimer (AF-M) and train linear models on physics-based energy terms to predict MP3-seq values. We find that AF-M-based models could be valuable for prescreening interactions but experimentally measuring interactions remains necessary to rank their strengths quantitatively. A method called massively parallel PPI measurement by sequencing (MP3-seq) is developed for measuring protein–protein interactions at scale. MP3-seq uses DNA barcodes that are associated with specific protein pairs and provides a quantitative measure of interaction strength. Interactions between rationally designed heterodimers and elements conferring interaction specificity were investigated using MP3-seq.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


