肠癌后的健康饮食和积极生活方式(HEAL ABC)--可行性随机对照试验。
IF 3.6
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
摘要
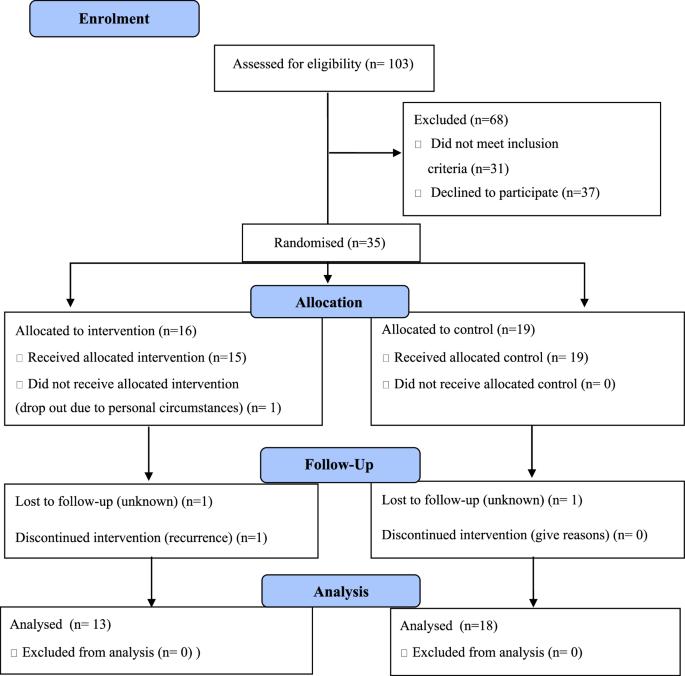
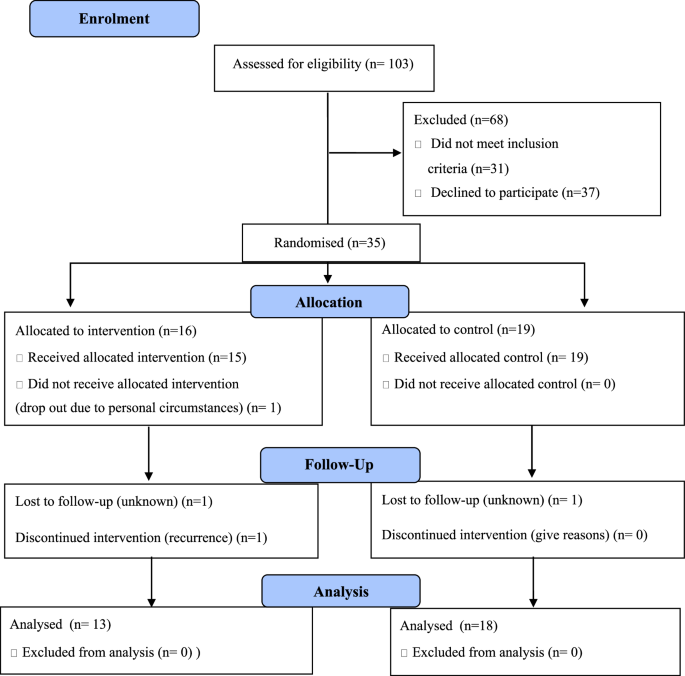
背景:队列研究的证据表明,健康的生活方式可以提高癌症患者的生存率,但缺乏随机对照试验(RCT)的证据。因此,本研究测试了对结肠直肠癌(CRC)治疗后的患者进行生活方式干预的可行性:方法:根据世界癌症研究基金会和美国癌症研究所(WCRF/AICR)的建议、健康行动过程方法和动机访谈法制定了一项干预措施,并进行了一项可行性混合方法 RCT 试验。参与者被分配到为期三个月的电话干预组和标准护理对照组。随访期为六个月。使用Stata(V15,StataCorp LLC)和NVivo 12(QSR International Pty Ltd.,Doncaster,VIC)收集并分析了可行性数据和次要结果:招募工作极具挑战性(31 人不符合条件,37 人拒绝;招募率 = 48.6%)。总共有 34/35 名参与者完成了干预,31 人(89%)完成了随访;所有 31 名完成者都在干预期间参加了六次电话访谈,并接受了六个月的随访。3 个月和 6 个月的研究保留率分别为 97%(34/35)和 89%(31/35)。数据完成率很高(>90%)。干预措施为参与者所接受,满足了他们的需求,并使他们对自己的目标负责。干预组的参与者在WCRF/AICR、国际膳食质量指数(Diet Quality Index-International)得分方面有显著改善,超加工食品的摄入量减少了10%:对 87% 的干预参与者来说,HEAL ABC 干预是可行的,能支持他们改变健康的生活方式。然而,要确定该干预措施的有效性,还需要采取其他招募策略,以进行全面的 RCT 研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Healthy Eating and Active Lifestyle after Bowel Cancer (HEAL ABC)—feasibility randomised controlled trial
Evidence from cohort studies indicates that a healthy lifestyle can improve cancer survival but evidence from randomised controlled trials (RCT) is lacking. Thus, this study tested the feasibility of conducting a lifestyle intervention in patients after colorectal cancer (CRC) treatment. An intervention was developed based on World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) recommendations, the Health Action Process Approach, Motivational Interviewing and tested a feasibility, mixed-methods RCT. Participants were allocated to a three-month telephone-based intervention versus standard care control group. The follow up period was six months. Data on feasibility and secondary outcomes were collected and analysed using Stata (V15, StataCorp LLC) and NVivo 12 (QSR International Pty Ltd., Doncaster, VIC). Recruitment was challenging (31 ineligible, 37 declined; recruitment rate = 48.6%.). In total, 34/35 participants completed the intervention, and 31 (89%) completed follow up; all 31 completers participated in six telephone calls during intervention and six months follow up. Study retention was 97% (34/35) and 89% (31/35) at three and six months, respectively. Data completion rates were high (>90%). Intervention was acceptable to participants, met their needs and kept them accountable towards their goals. Participants in the intervention group showed significant improvement in WCRF/AICR, Diet Quality Index-International score and a 10% reduction in ultra-processed food consumption. The HEAL ABC intervention was feasible for 87% of intervention participants, supporting them in healthy lifestyle changes. However, alternative recruitment strategies are needed for a fully powered RCT to determine the effectiveness of the intervention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.60
自引率
2.10%
发文量
189
审稿时长
3-6 weeks
期刊介绍:
The European Journal of Clinical Nutrition (EJCN) is an international, peer-reviewed journal covering all aspects of human and clinical nutrition. The journal welcomes original research, reviews, case reports and brief communications based on clinical, metabolic and epidemiological studies that describe methodologies, mechanisms, associations and benefits of nutritional interventions for clinical disease and health promotion.
Topics of interest include but are not limited to:
Nutrition and Health (including climate and ecological aspects)
Metabolism & Metabolomics
Genomics and personalized strategies in nutrition
Nutrition during the early life cycle
Health issues and nutrition in the elderly
Phenotyping in clinical nutrition
Nutrition in acute and chronic diseases
The double burden of ''malnutrition'': Under-nutrition and Obesity
Prevention of Non Communicable Diseases (NCD)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


