设计阿昔洛韦诱导的 RNA 纳米器件,实现对适配体功能的可逆和可调控制。
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
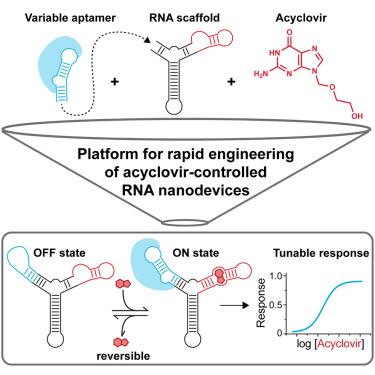
小分子调控的 RNA 装置有可能调节细胞功能的各个方面,但迄今为止使用的小分子具有潜在毒性,限制了它们在细胞中的使用。在这里,我们介绍了一种利用阿昔洛韦(一种生物相容性极好、毒性极低的小分子)制造药物调控 RNA 纳米器件(RN)的方法。我们的模块化方法包括一个由中央 F30 三向接头、输入臂上的集成阿昔洛韦适配体和输出臂上的可变效应物结合适配体组成的支架。这种设计可以快速设计阿昔洛韦调控的 RN,促进对细胞内适配体的时间性、可调性和可逆性控制。我们展示了阿昔洛韦对 Broccoli aptamer 和铁反应元件 (IRE) 的控制。用阿昔洛韦调控 IRE 可以精确控制铁调节蛋白(IRP)的螯合,从而促进对铁突变的抑制。总之,本文介绍的方法为将适配体转化为阿昔洛韦可控的生理靶蛋白拮抗剂提供了一个平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineering acyclovir-induced RNA nanodevices for reversible and tunable control of aptamer function
Small molecule-regulated RNA devices have the potential to modulate diverse aspects of cellular function, but the small molecules used to date have potential toxicities limiting their use in cells. Here we describe a method for creating drug-regulated RNA nanodevices (RNs) using acyclovir, a biologically compatible small molecule with minimal toxicity. Our modular approach involves a scaffold comprising a central F30 three-way junction, an integrated acyclovir aptamer on the input arm, and a variable effector-binding aptamer on the output arm. This design allows for the rapid engineering of acyclovir-regulated RNs, facilitating temporal, tunable, and reversible control of intracellular aptamers. We demonstrate the control of the Broccoli aptamer and the iron-responsive element (IRE) by acyclovir. Regulating the IRE with acyclovir enables precise control over iron-regulatory protein (IRP) sequestration, consequently promoting the inhibition of ferroptosis. Overall, the method described here provides a platform for transforming aptamers into acyclovir-controllable antagonists against physiologic target proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


