TIGIT-LAG3阻断治疗骨髓瘤后的临床反应和通路特异性相关性:MyCheckpoint随机临床试验。
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
骨髓瘤患者被随机分配接受抗TIGIT(T细胞免疫受体)或抗LAG3(淋巴细胞活化基因)抗体,然后联合使用泊马度胺和地塞米松(NCT04150965)。主要和次要终点分别为安全性和有效性。治疗耐受性良好,无剂量限制性毒性。抗TIGIT治疗组(6名参与者中的3名)和抗LAG3治疗组(6名参与者中的2名)都观察到了持久的临床反应。抗LAG3应答者的幼稚分化4群(CD4)阳性T细胞较高,而程序性细胞死亡蛋白1阳性效应T细胞较低。抗TIGIT应答者的CD226表达和自然杀伤细胞活化程度较高,CD112表达较低。这些数据证明了TIGIT-LAG3阻断剂的临床活性,并确定了骨髓瘤中特异性反应途径的相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
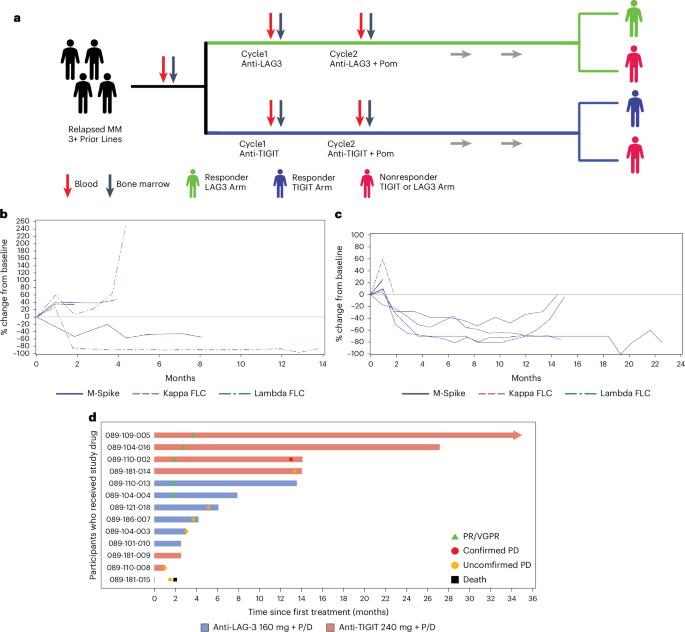
Clinical response and pathway-specific correlates following TIGIT–LAG3 blockade in myeloma: the MyCheckpoint randomized clinical trial
Persons with myeloma were randomized to receive an anti-TIGIT (T cell immunoreceptor) or anti-LAG3 (lymphocyte activation gene) antibody followed by combination with pomalidomide and dexamethasone ( NCT04150965 ). Primary and secondary endpoints were safety and efficacy, respectively. Therapy was well tolerated without dose-limiting toxicity. Durable clinical responses were observed in both the anti-TIGIT(three of six participants) and the anti-LAG3 (two of six participants) arms. Anti-LAG3 responders had higher naive cluster of differentiation 4 (CD4)-positive T cells and lower programmed cell death protein 1-positive effector T cells. Anti-TIGIT responders had higher CD226 expression, natural killer cell activation and lower CD112 expression. These data demonstrate the clinical activity of TIGIT–LAG3 blockade and identify pathway-specific response correlates in myeloma. Richard et al. perform a clinical trial of anti-TIGIT (T cell immunoreceptor) or anti-LAG3 (lymphocyte activation gene) antibody in combination with pomalidomide and dexamethasone in persons with multiple myeloma and define correlates of response using mass cytometry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


