金属离子螯合苯丙氨酸纳米结构可逆转免疫功能障碍,并使乳腺肿瘤对免疫检查点阻断剂敏感。
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
免疫抑制性肿瘤微环境会严重影响接受基于免疫检查点阻断剂的癌症免疫疗法(如程序性死亡-1(PD-1)和程序性死亡配体1(PD-L1))的患者的应答率。在这里,我们证明了金属离子螯合 L-苯丙氨酸纳米结构与短期饥饿(STS)协同重塑了乳腺和结直肠肿瘤的免疫抑制微环境。这些纳米结构可调节树突状细胞的电生理行为,并通过 NLRP3 炎性体和钙介导的核因子-κB 途径激活树突状细胞。STS 通过氨基酸转运体促进细胞对纳米结构的吸收,并在树突状细胞成熟和肿瘤特异性细胞毒性 T 淋巴细胞反应中发挥关键作用。这项研究证明了金属离子螯合 L-苯丙氨酸纳米结构在激活免疫反应方面的潜在作用,以及 STS 处理在改善纳米材料介导的癌症免疫疗法方面的效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。

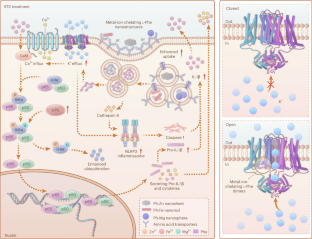
Metal-ion-chelating phenylalanine nanostructures reverse immune dysfunction and sensitize breast tumour to immune checkpoint blockade
An immunosuppressive tumour microenvironment strongly influences response rates in patients receiving immune checkpoint blockade-based cancer immunotherapies, such as programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1). Here we demonstrate that metal-ion-chelating l-phenylalanine nanostructures synergize with short-term starvation (STS) to remodel the immunosuppressive microenvironment of breast and colorectal tumours. These nanostructures modulate the electrophysiological behaviour of dendritic cells and activate them through the NLRP3 inflammasome and calcium-mediated nuclear factor-κB pathway. STS promotes the cellular uptake of nanostructures through amino acid transporters and plays a key role in dendritic cell maturation and tumour-specific cytotoxic T lymphocyte responses. This study demonstrates the potential role of metal-ion-chelating l-phenylalanine nanostructures in activating immune responses and the effect of STS treatment in improving nanomaterial-mediated cancer immunotherapy. Metal-ion-chelating phenylalanine nanostructures modulate ion influx and efflux in dendritic cells, activating them through the NLRP3 inflammasome and NF-κB pathway to remodel the immunosuppressive tumour microenvironment for PD-L1-based immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


