基于甲醛促进偶氮基氨基甲酸酯的 C-N 裂解的甲醛荧光探针。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
甲醛(FA)是一种内源性单碳代谢物,也是一种环境污染物和致癌物质。甲醛水平升高与许多疾病相关。便捷的原位检测 FA 水平的方法对于了解 FA 的生物功能和信号通路具有重要意义。在此,我们开发了用于检测 FA 的 NAP-FAP2 系列荧光探针,该探针基于 FA 通过 FA 诱导的分子内作用促进 3-硝基苯基氮杂酰 N-芳基氨基甲酸酯的 C-N 裂解,其中芳基是荧光团 1,8-萘二甲酰亚胺-4-基。含有活性基团的 3-硝基苯基氮杂环胺还通过光诱导电子传递机制作为荧光淬灭基团,在与 FA 反应后产生开启荧光响应。应用这些探针探讨了麦拉宁诱导的铁中毒中 FA 水平的变化,结果发现,FA 水平在细胞内增加,而在内质网中没有增加,这表明铁中毒中 FA 水平的增加并非来自脂质过氧化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
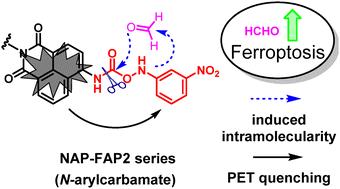
Fluorescent probes for formaldehyde based on formaldehyde-promoted C–N cleavage of azanyl carbamates†
Formaldehyde (FA) is an endogenous one-carbon metabolite and an environmental pollutant and carcinogen. Elevated FA levels are associated with many diseases. Methods for the convenient and in situ detection of FA levels are of great significance for understanding FA's biofunctions and signalling pathways. Herein, the NAP-FAP2 series of fluorescent probes for FA detection were developed based on FA-promoted C–N cleavage of 3-nitrophenylazanyl N-arylcarbamate via FA-induced intramolecularity, where the aryl group is the fluorophore 1,8-naphthalimide-4-yl. The 3-nitrophenylazanyl containing reactive group also functions as a fluorescence quenching group via a photo-induced electron transfer mechanism to generate turn-on fluorescence response upon reaction with FA. The probes were applied to explore FA level changes in erastin-induced ferroptosis, and it was found that the FA level increases intracellularly, but not in the endoplasmic reticulum, suggesting that the FA level increases in ferroptosis are not derived from lipid peroxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


