多光子激发聚合胶原纤维形态的仿生模型,用于研究胰腺癌的单细胞和集体迁移动力学。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
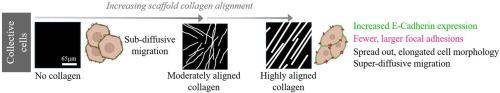
在胰腺癌患者的细胞外基质(ECM)中发现的排列整齐的胶原纤维形态和细胞迁移动力学的各自作用越来越受到关注,因为它们与侵袭性表型增加和预后不良有关。为了更好地了解胶原纤维形态如何影响与转移相关的细胞-基质相互作用,我们利用胰腺导管腺癌(PDAC)患者活检组织的二次谐波发生(SHG)图像作为模型,制作胶原支架来研究与运动相关的过程。我们利用 PDAC BxPC-3 转移细胞系,研究了不同胶原排列的支架上单细胞和集群细胞的动态。与单个细胞相比,生长在胶原纤维排列最高的支架上的集体或集群细胞的E-cadherin表达增加,病灶粘附点增大,这与转移行为一致。对单细胞运动的分析表明,在所有基质上的动态特征都是随机行走。然而,对不同时间点的集体运动进行研究后发现,在高度排列的纤维上,迁移具有超扩散性并得到增强,而在无图案的基质上,迁移则受到阻碍并呈次扩散性。在排列整齐的胶原纤维上观察到的集体迁移细胞形态更加细长,进一步证实了这一点。总之,这种方法可以将单细胞行为和集体细胞行为作为胶原排列的函数进行解耦,并显示了集体细胞行为和纤维形态在 PDAC 转移中的相对重要性。我们建议将这些支架用于进一步研究 PDAC 细胞生物学。意义声明:胰腺导管腺癌(PDAC)的死亡率很高,其中排列整齐的胶原蛋白与不良预后有关。要了解复杂的细胞相互作用,就需要代表这种结构的仿生模型。基于人体活检组织基质胶原的 SHG 图像模型可以测量细胞形态、粘附蛋白和局灶粘附表达以及详细的运动动态。利用转移细胞系,我们将单细胞行为和细胞集体行为的作用以及排列胶原蛋白的作用分离开来。我们的数据表明,胶原排列的增加增强了转移特性,而细胞的集体行为与转移过程更为相关。这些支架为了解这种疾病提供了新的视角,可作为进一步实验(如测试药物疗效)的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multiphoton excited polymerized biomimetic models of collagen fiber morphology to study single cell and collective migration dynamics in pancreatic cancer
The respective roles of aligned collagen fiber morphology found in the extracellular matrix (ECM) of pancreatic cancer patients and cellular migration dynamics have been gaining attention because of their connection with increased aggressive phenotypes and poor prognosis. To better understand how collagen fiber morphology influences cell-matrix interactions associated with metastasis, we used Second Harmonic Generation (SHG) images from patient biopsies with Pancreatic ductal adenocarcinoma (PDAC) as models to fabricate collagen scaffolds to investigate processes associated with motility. Using the PDAC BxPC-3 metastatic cell line, we investigated single and collective cell dynamics on scaffolds of varying collagen alignment. Collective or clustered cells grown on the scaffolds with the highest collagen fiber alignment had increased E-cadherin expression and larger focal adhesion sites compared to single cells, consistent with metastatic behavior. Analysis of single cell motility revealed that the dynamics were characterized by random walk on all substrates. However, examining collective motility over different time points showed that the migration was super-diffusive and enhanced on highly aligned fibers, whereas it was hindered and sub-diffusive on un-patterned substrates. This was further supported by the more elongated morphology observed in collectively migrating cells on aligned collagen fibers. Overall, this approach allows the decoupling of single and collective cell behavior as a function of collagen alignment and shows the relative importance of collective cell behavior as well as fiber morphology in PDAC metastasis. We suggest these scaffolds can be used for further investigations of PDAC cell biology.
Statement of significance
Pancreatic ductal adenocarcinoma (PDAC) has a high mortality rate, where aligned collagen has been associated with poor prognosis. Biomimetic models representing this architecture are needed to understand complex cellular interactions. The SHG image-based models based on stromal collagen from human biopsies afford the measurements of cell morphology, cadherin and focal adhesion expression as well as detailed motility dynamics. Using a metastatic cell line, we decoupled the roles of single cell and collective cell behavior as well as that arising from aligned collagen. Our data suggests that metastatic characteristics are enhanced by increased collagen alignment and that collective cell behavior is more relevant to metastatic processes. These scaffolds provide new insight in this disease and can be a platform for further experiments such as testing drug efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


