BRD-810 是一种高选择性 MCL1 抑制剂,具有优化的体内清除率,在实体瘤和血液肿瘤模型中疗效显著。
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
MCL1 基因在癌症中经常被扩增,它编码抗凋亡蛋白髓系细胞白血病 1(MCL1),该蛋白对目前的治疗标准具有抗药性。因此,MCL1 是一个极具吸引力的抗癌靶点。在此,我们介绍了作为一种强效、选择性 MCL1 抑制剂的 BRD-810,以及它的关键设计原则--快速全身清除,从而最大限度地降低与 MCL1 抑制相关的曲线下面积毒性。BRD-810能在体外4小时内快速杀死细胞,但在同一4小时窗口期内,即使在超药物浓度下,也不会影响细胞存活率或肌钙蛋白I在人诱导多能干细胞衍生心肌细胞中的释放。尽管BRD-810在血浆中的停留时间很短,但在体内BRD-810却能诱导异种移植血液肿瘤和实体肿瘤模型产生疗效。总之,我们的数据支持这样的假设,即用 BRD-810 短期抑制 MCL1 可以诱导肿瘤细胞凋亡,同时保持可接受的安全性。因此,我们打算将 BRD-810 推向临床试验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
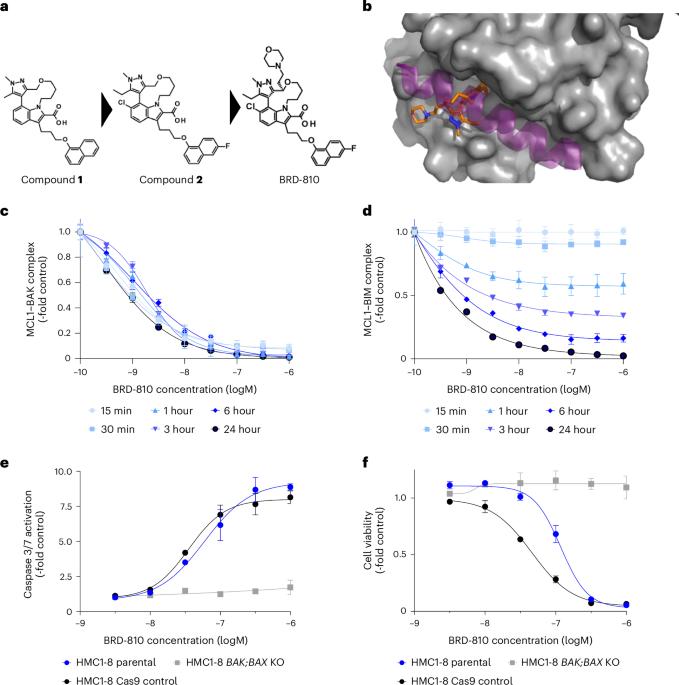
BRD-810 is a highly selective MCL1 inhibitor with optimized in vivo clearance and robust efficacy in solid and hematological tumor models
The MCL1 gene is frequently amplified in cancer and codes for the antiapoptotic protein myeloid cell leukemia 1 (MCL1), which confers resistance to the current standard of care. Therefore, MCL1 is an attractive anticancer target. Here we describe BRD-810 as a potent and selective MCL1 inhibitor and its key design principle of rapid systemic clearance to potentially minimize area under the curve-driven toxicities associated with MCL1 inhibition. BRD-810 induced rapid cell killing within 4 h in vitro but, in the same 4-h window, had no impact on cell viability or troponin I release in human induced pluripotent stem cell-derived cardiomyocytes, even at suprapharmacologic concentrations. In vivo BRD-810 induced efficacy in xenograft hematological and solid tumor models despite the short residence time of BRD-810 in plasma. In totality, our data support the hypothesis that short-term inhibition of MCL1 with BRD-810 can induce apoptosis in tumor cells while maintaining an acceptable safety profile. We, therefore, intend to advance BRD-810 to clinical trials. Rauh et al. developed a selective MCL1 inhibitor that is efficacious in hematological and solid tumors and has the advantage of limited cardiotoxicity because of more rapid clearance of the drug in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


