在介孔 HZSM-5 和 Ga/ZSM-5 催化剂上 CO2 辅助催化热解聚烯烃制芳烃
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
催化热解被认为是将聚烯烃转化为高价值芳烃以实现循环经济的潜在途径。然而,沸石固有的扩散限制和催化热解过程中过量氢的形成限制了芳烃的选择性。在本研究中,我们提出构建介孔 Ga 改性 HZSM-5,以消除扩散限制并改善芳烃的形状选择催化。同时,我们采用二氧化碳作为温和的氧化剂来去除过量的氢气,以加速中间烷烃和烯烃的芳构化。利用二氧化碳与 Ga/ZSM-5-meso 酸性位点之间的协同效应,使 BTEX 含量超过 60%。与 N2 气氛相比,CO2 能显著提高所有催化剂(HZSM-5、HZSM-5-meso、Ga/ZSM-5 和 Ga/ZSM-5-meso)的芳烃产率。值得注意的是,Ga-Lewis 酸位点和介孔结构可促进脱氢反应以产生 H2,H2 随后可通过反向水气变换 (RWGS) 和甲烷化反应消耗掉,从而加速了芳烃生产的平衡。在这种情况下,气体中的大部分烯烃和石油中的非芳烃会完全转化为芳烃。与 N2-HZSM-5 的 40.7% 和 96.2% 相比,CO2-Ga-ZSM-5-meso 的相应产油量和芳烃选择性分别高达 63.3% 和 100%。此外,CO2 还具有脱烷基的作用,导致苯和甲苯的相对含量显著增加。在气体成分中,H2 的含量明显减少,而 CH4 的含量增加,并产生 CO。最后,提出了在 Ga/ZSM-5-meso 上 CO2- 辅助催化热解聚烯烃的协同催化机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
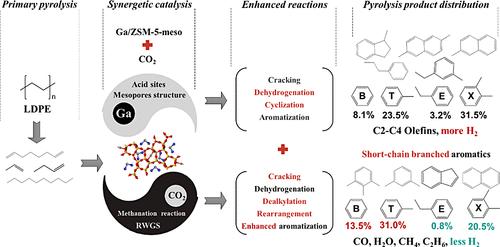
CO2-Assisted Catalytic Pyrolysis of Polyolefins to Aromatics over Mesoporous HZSM-5 and Ga/ZSM-5 Catalysts
Catalytic pyrolysis is considered as a potential route to convert polyolefins to high-value aromatics toward a circular economy. However, the inherent diffusional constraints of zeolites and the formation of excess hydrogen during catalytic pyrolysis limit the selectivity of aromatics. In this study, we propose to construct mesoporous Ga-modified HZSM-5 to eliminate diffusion restriction and improve shape selection catalysis of aromatics. At the same time, we adopt CO2 as a mild oxidizer to remove excessive hydrogen to expedite the aromatization of intermediate alkanes and olefins. Developing the synergistic effect between CO2 and acid sites of Ga/ZSM-5-meso makes the BTEX content exceed 60%. Compared with the N2 atmosphere, CO2 significantly enhances the yield of aromatics for all of the catalysts (HZSM-5, HZSM-5-meso, Ga/ZSM-5, and Ga/ZSM-5-meso). Significantly, Ga-Lewis acid sites and mesoporous structures promote dehydrogenation to produce H2, which can be subsequently consumed through reverse water gas shift (RWGS) and methanation reactions, accelerating the equilibrium toward aromatics production. In this case, most of olefins in gas and nonaromatic hydrocarbons (non-AHs) in oil are completely converted into aromatics. Corresponding oil yield and aromatics selectivity in oil reach as high as 63.3 and 100% for CO2−Ga-ZSM-5-meso, respectively, compared with 40.7 and 96.2% for N2−HZSM-5. Moreover, CO2 has the effect of dealkylation, leading to a notable increase in the relative contents of benzene and toluene. In gas composition, the content of H2 decreases obviously while the content of CH4 increases, and CO is produced. Finally, the synergetic catalysis mechanism of the CO2-assisted catalytic pyrolysis of polyolefins over Ga/ZSM-5-meso is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


