通过将生物合成杂环酶整合到多酶级联中,实现手性饱和氧杂环的高立体选择性生物催化一锅合成
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
次级代谢是具有新合成活性的酶的丰富来源,这些酶尚未被纳入生物催化工具箱。手性饱和氧杂环(CSOH)是天然产品和其他高附加值化合物的丰富结构元素。我们介绍了一种利用容易获得的前体合成 CSOHs 的生物催化方法,该方法将生物合成途径中的分子内氧杂迈克尔加成(IMOMA)催化环化酶(CYC)与醇脱氢酶(ADH)和硫酯衍生酶结合在一起。单锅 ADH-CYC 反应能够在多达四个立体中心的控制下获得各种四氢吡喃(THP)和四氢呋喃硫酯。通过化学和酶法手段,这些产物很容易转化为有用的 CSOH 酮、酰胺、醛/醇、酯和羧酸构建模块。通过添加硫代酯酶和羧酸还原酶,可以直接通过化学酶法合成天然产物 (-)-civet、一种新的衍生物和一种 THP 醇,这证明了多酶级联可以扩展到更复杂的多酶级联。将 IMOMA 环化酶整合到酶级联中,可以更好地利用这组新的成环酶的高合成潜力,并作为一种高选择性和多功能的替代方法,扩大了合成药理相关 CSOH 的范围。这种方法可以通过改变 ADH、IMOMA 环化酶和修饰酶来合成多种 CSOH。本文章由计算机程序翻译,如有差异,请以英文原文为准。
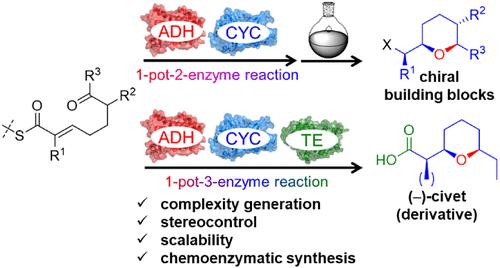
Highly Stereoselective Biocatalytic One-Pot Synthesis of Chiral Saturated Oxygen Heterocycles by Integration of a Biosynthetic Heterocyclase into Multiple-Enzyme Cascades
The secondary metabolism is a rich source of enzymes with new synthetically attractive activities that have not yet been integrated into the toolbox of biocatalysis. Chiral saturated oxygen heterocycles (CSOHs) are abundant structural elements of natural products and other value-added compounds. We present a biocatalytic method for the synthesis of CSOHs from readily accessible precursors that combines an intramolecular oxa-Michael addition (IMOMA)-catalyzing cyclase (CYC) from a biosynthetic pathway with alcohol dehydrogenases (ADHs) and thioester-derivatizing enzymes. The one-pot ADH–CYC reaction enables access to various tetrahydropyran (THP) and tetrahydrofuran thioesters under control of up to four stereocenters. These products are readily convertible into useful CSOH ketone, amide, aldehyde/alcohol, ester, and carboxylic acid building blocks by chemical and enzymatic means. The extendibility to more complex multienzyme cascades was demonstrated by the addition of a thioesterase and a carboxylic acid reductase, allowing the straightforward chemoenzymatic synthesis of the natural product (−)-civet, a new derivative, and a THP alcohol. The integration of IMOMA cyclases into enzymatic cascades allows better exploitation of the high synthetic potential of this new group of ring-forming enzymes and expands the repertoire for the synthesis of pharmacologically relevant CSOHs as a highly selective and versatile alternative. This approach will be adaptable for the synthesis of a wide range of CSOHs by varying ADHs, IMOMA cyclases, and modifying enzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


