长期抗逆转录病毒疗法后的克隆继承可使 CD8+ T 细胞对 HIV-1 的反应恢复活力
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
人类免疫缺陷病毒 1(HIV-1)感染的特点是病毒复制的动态和持续状态,在没有抗逆转录病毒疗法(ART)的情况下,病毒复制会使宿主免疫系统不堪重负。然而,长期治疗对 HIV-1 特异性 CD8+ T 细胞抗病毒功效的影响仍然未知。在这里,我们利用单细胞技术在大流行期间早期感染并随后接受持续抗逆转录病毒疗法治疗的高龄人群中解决了这一问题。我们的数据显示,长期抗逆转录病毒疗法与克隆继承过程有关,面对免疫衰老,它能有效地使 HIV-1 特异性 CD8+ T 细胞群恢复活力。对个体转录组的追踪进一步显示,最初占优势的CD8+ T细胞克隆型显示出衰竭和终末分化的特征,而新占优势的CD8+ T细胞克隆型则显示出早期分化和干性的特征,这与病毒复制的自然控制有关。这些发现揭示了一定程度的免疫恢复能力,可为HIV-1的辅助治疗提供依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Clonal succession after prolonged antiretroviral therapy rejuvenates CD8+ T cell responses against HIV-1
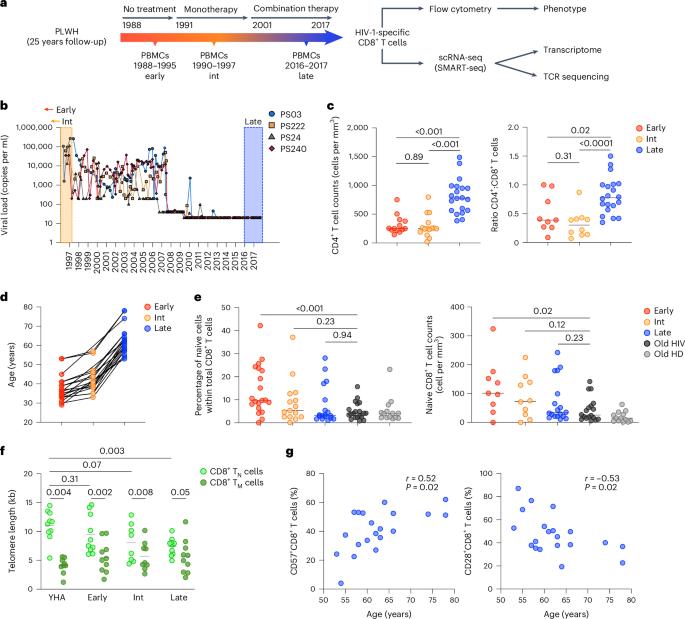
Human immunodeficiency virus 1 (HIV-1) infection is characterized by a dynamic and persistent state of viral replication that overwhelms the host immune system in the absence of antiretroviral therapy (ART). The impact of prolonged treatment on the antiviral efficacy of HIV-1-specific CD8+ T cells has nonetheless remained unknown. Here, we used single-cell technologies to address this issue in a cohort of aging individuals infected early during the pandemic and subsequently treated with continuous ART. Our data showed that long-term ART was associated with a process of clonal succession, which effectively rejuvenated HIV-1-specific CD8+ T cell populations in the face of immune senescence. Tracking individual transcriptomes further revealed that initially dominant CD8+ T cell clonotypes displayed signatures of exhaustion and terminal differentiation, whereas newly dominant CD8+ T cell clonotypes displayed signatures of early differentiation and stemness associated with natural control of viral replication. These findings reveal a degree of immune resilience that could inform adjunctive treatments for HIV-1. Appay and colleagues show that long-term antiretroviral therapy is associated with clonal succession of HIV-1-specific CD8+ T cell populations, which evolved from an exhaustion-like toward a stemness-like phenotype.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


