使用具有正交改性 1,10- 苯并脯氨酸配体的超高活性铁(II)络合物催化异戊二烯聚合,获得热塑橡胶
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
过去几十年来,双齿 N,N-配体在铁介导的 1,3-二烯聚合中发挥了重要作用。在这项工作中,合成并表征了螯合有 1,10- 苯并脯氨酸配体的铁配合物,该配体在 2 位上修饰了电子捐赠(硫)醚和二乙胺取代基。供体的存在使异戊二烯聚合的催化活性提高了 1 倍,达到了迄今为止发现的最活跃的铁体系(2.60×107g-mol-1-h-1)。在零下 40 摄氏度的条件下,3,4 选择性高达 69.9%,同时具有适度的联合能力(rr:60.2%)。活性与杂原子有关,催化剂含醚(1.20×107g-mol-1-h-1-0.96×107g-mol-1-h-1)比噻吩醚(Fe5,0.89×107g-mol-1-h-1)和二乙胺(Fe6,0.67×107g-mol-1-h-1)类似物的活性更高。在不同温度下合成的聚异戊二烯经压制和模塑后,形成了一种新型材料,具有出色的综合强度(断裂强度为 15.6 兆帕)和韧性(伸长率高达 589%)。热可塑性使其具有良好的再加工性,在三个周期后性能恢复令人满意。本文章由计算机程序翻译,如有差异,请以英文原文为准。
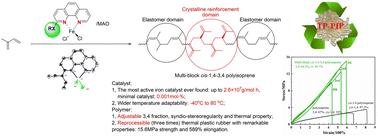
Catalytic polymerization of isoprene using an ultrahigh active iron(ii) complex with an ortho-modified 1,10-phenanthroline ligand, access to a thermal plastic rubber†
Bidentate N,N-ligands have played a vital role in the iron-mediated polymerization of 1,3-diene over the past few decades. In this work, iron complexes chelated with a 1,10-phenanthroline ligand modified with an electronic donating (thio)ether, diethylamine substituents at the 2-position, were synthesized and characterized. The presence of the donor promoted the catalytic activity by one-fold for isoprene polymerization, reaching the most active iron system (2.60 × 107 g mol−1 h−1) ever found so far. An elevated 3,4 selectivity of 69.9% with moderate syndiotacticity (rr: 60.2%) was achieved at −40 °C. The activity was heteroatom-dependent, with the catalyst bearing ether (1.20 × 107 g mol−1 h−1 –0.96 × 107 g mol−1 h−1) performing more actively than thiophenyl ether (Fe5, 0.89 × 107 g mol−1 h−1) and diethylamine (Fe6, 0.67 × 107 g mol−1 h−1) analogues. Polyisoprenes synthesized under various temperatures were pressed and molded to afford a new type of material with integrated excellent strength (breaking strength, 15.6 MPa) and toughness (elongation up to 589%). The thermal plasticity granted a good reprocessability with satisfactory property recovery after three cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


