利用催化不对称 Suzukii-Miyaura 交叉偶联构建轴向手性 2-arylpyrroles: 一种高效的艾沙烯酮制备方法。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用催化不对称铃木-宫浦交叉偶联,开发了一种获得轴向手性 2-芳基吡咯的通用高效方法。该方法获得了多种轴向手性芳基吡咯,产量高,对映体选择性好到极佳。成功的关键在于使用了一个包括钯催化剂和手性二茂铁二膦配体的组合催化体系,从而实现了有效的对映控制。更重要的是,这种轴向手性 CF3 取代的 2-芳基吡咯是制备抗高血压和糖尿病肾病药物埃沙塞酮的关键中间体。该化合物以高对映选择性(92% ee)直接不对称合成。因此,这为催化不对称合成艾沙西仑酮提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
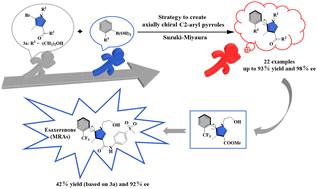
Construction of axially chiral 2-arylpyrroles using catalytic asymmetric Suzuki–Miyaura cross-coupling: an efficient approach to esaxerenone†
A general and efficient method has been developed to access axially chiral 2-arylpyrroles using catalytic asymmetric Suzuki–Miyaura cross-coupling. A wide range of axially chiral arylpyrroles were obtained in high yields with good to excellent enantioselectivities. The key to success is the use of a combined catalytic system involving a palladium catalyst and chiral ferrocene diphosphine ligand for achieving effective enantiocontrol. More importantly, this axially chiral CF3-substituted 2-arylpyrrole serves as a key intermediate in the preparation of the anti-hypertensive and diabetic nephropathy drug esaxerenone. It was directly asymmetrically synthesized with high enantioselectivity (92% ee). Thus, a new strategy is provided for the catalytic asymmetric synthesis of esaxerenone.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


