RNA 寡聚体中安装表转录组 5 修饰胞嘧啶的化学原理。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
作为细胞 mRNA 中 5-甲基胞苷(m5C)氧化去甲基化途径的产物,对 5-羟甲基胞苷(hm5C)、5-甲酰基胞苷(f5C)和 5-羧基胞苷(ca5C)修饰的研究构成了新的表转录组学研究领域的重要内容。m5C 转换并最终转化为母胞苷的动态过程被认为是基因表达调控的转录后层次。然而,与外转录组胞苷修饰相关的调控机制在很大程度上仍是未知的。因此,含有 m5C 氧化产物的寡核苷酸对于下一代生化、生物物理和结构研究其功能、代谢和对人类疾病的影响具有重要价值。在此,我们总结了通过磷酰胺化学将 hm5C、f5C 和 ca5C 加入 RNA 寡聚体的合成策略,包括合成后 C5-胞苷功能化和酶解方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
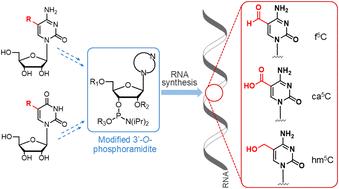
Chemistry of installing epitranscriptomic 5-modified cytidines in RNA oligomers
Studies of 5-hydroxymethylcytidine (hm5C), 5-formylcytidine (f5C) and 5-carboxycytidine (ca5C) modifications as products of the 5-methylcytidine (m5C) oxidative demethylation pathway in cellular mRNAs constitute an important element of the new epitranscriptomic field of research. The dynamic process of m5C conversion and final turnover to the parent cytidine is considered a post-transcriptional layer of gene-expression regulation. However, the regulatory mechanism associated with epitranscriptomic cytidine modifications remains largely unknown. Therefore, oligonucleotides containing m5C oxidation products are of great value for the next generation of biochemical, biophysical, and structural studies on their function, metabolism, and contribution to human diseases. Herein, we summarize the synthetic strategies developed for the incorporation of hm5C, f5C and ca5C into RNA oligomers by phosphoramidite chemistry, including post-synthetic C5-cytidine functionalization and enzymatic methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


