由二甲基亚砜和 1,2-二卤乙烷生成的 "阳离子池 "及其在有机合成中的应用。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
由于硒和锇离子的不稳定性和瞬时性,它们通常是在有亲核物存在的情况下制备的。对反应介质不稳定或惰性的亲核物不能用于与阳离子物种发生反应,以获得所需的化合物。为了克服这些限制,必须开发出在无亲核物的情况下不可逆地生成有机阳离子的方法。Yoshida 及其合作者开发的 "阳离子池 "方法是一种可靠的策略,可以在没有亲核物的情况下在溶液中生成和积累活性阳离子。阳离子池法涉及在无亲核物的情况下,通常在低温下对底物进行电解。此外,通过电解生成卤素阳离子和铬阳离子需要格外小心,因为它们的稳定性较低。本综述介绍了我们在生成和积累卤素阳离子作为 "阳离子池 "方面所做的努力,其中最重要的是通过简单加热二甲基亚砜(DMSO)和 1,2-二卤乙烷(DXE,X = Cl、Br、I)的混合物,以及它们在卤化反应中的应用。此外,还介绍了 DMSO 稳定卤素阳离子在合成应用中与条件相关的 Pummerer 型片段生成甲基(亚甲基)锍离子和氯二甲基锍离子的情况。本文章由计算机程序翻译,如有差异,请以英文原文为准。
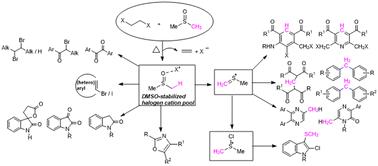
“Cation Pool” generated from DMSO and 1,2-dihaloethanes and their application in organic synthesis
Conventionally, carbenium and onium ions are prepared in the presence of nucleophiles due to their instability and transient nature. The nucleophiles that are unstable or inert to the reaction media cannot be used for reaction with the cationic species to access the desired compounds. To overcome these limitations, developing methods for generating organic cations irreversibly in the absence of nucleophiles is essential. The “cation pool” method developed by Yoshida and co-workers stands out as a reliable strategy to generate and accumulate the reactive cations in solution in the absence of nucleophiles. The cation pool method involves the electrolysis of the substrate in the absence of nucleophiles, usually at low temperature. Moreover, the generation of halogen and chalcogen cations through electrolysis needs extra care because of their low stability. This review covers our effort in generating and accumulating halogen cations as “cation pools”, most importantly by simply heating a mixture of dimethyl sulfoxide (DMSO) and 1,2-dihaloethane (DXE, X = Cl, Br, I), and their use in the halogenation reactions. Furthermore, condition-dependent Pummerer-type fragmentations of DMSO-stabilized halogen cations to methyl(methylene)sulfonium ions and chlorodimethylsulfonium ions for synthetic applications are described.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


