500 Wh kg-1 锂金属袋电池的振荡溶解化学反应
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
人们对阳离子溶解在溶液体相中的作用了解甚多,但对电解质-电解质界面的了解却很有限。阳离子溶解符合界面场以形成相间的过程仍不清楚。在此,我们研究了外部场和分子内场对锂金属阳极容纳 Li+ 溶解物的协同效应,从而导致界面上介电介质介导的转移动力学。在带电界面上,阳离子-阴离子对呈周期性振荡分布。低振荡振幅会加剧电解质分解并增加表面阻抗。为了解决这些问题,我们提出了一种电介质协议,它能在界面上以高振荡幅度保持阳离子-阴离子配位。因此,我们利用超低电解质(1 g Ah-1)演示了能量密度为 500 Wh kg-1 的锂金属袋电池。我们的研究为固态/液态界面提供了深入的见解,这对推动电池技术的发展至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
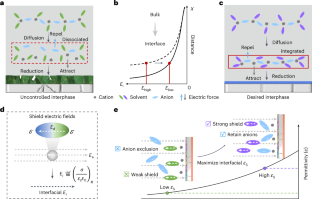
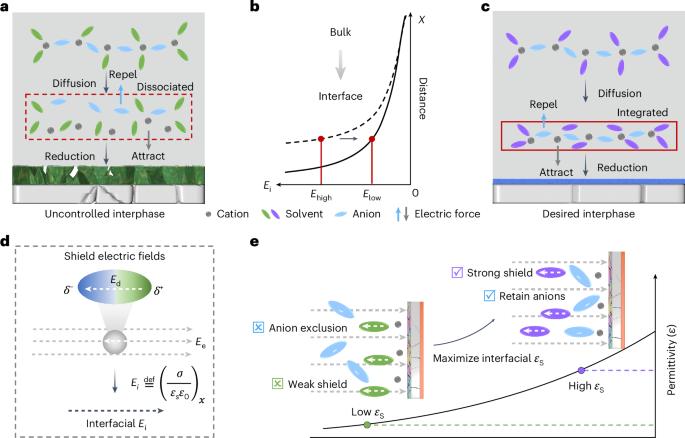
Oscillatory solvation chemistry for a 500 Wh kg−1 Li-metal pouch cell
Cation solvation is well understood in the bulk solution phase, but knowledge is limited regarding the electrode–electrolyte interface. The process by which cation solvation conforms to the interfacial field to form interphases remains unclear. Here we examine the synergistic effects of external and intramolecular fields on accommodating Li+ solvates to the Li-metal anode, leading to dielectric-mediated transfer dynamics on the interface. At charged interfaces, cation–anion pairs arrange in a periodic oscillatory distribution. A low-oscillation amplitude exacerbates the electrolyte decomposition and increases surface impedance. We propose a dielectric protocol that maintains cation–anion coordination with a high oscillation amplitude at the interfaces, addressing these issues. Accordingly, we demonstrate a Li-metal pouch cell with an energy density of 500 Wh kg−1 at the Ah level using an ultra-lean electrolyte (1 g Ah−1). Our study offers insights into solid/liquid interfaces that are crucial in advancing battery technologies. Cation solvation in batteries is well understood in bulk solutions but less so at electrode/electrolyte interfaces. This study reveals how external and intramolecular fields affect Li-ion solvation, proposing a dielectric protocol to enhance cation–anion coordination and improve performance in Li-metal pouch cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


