寻找 HLA-B∗27 为何会导致脊柱关节炎风险的生化、微生物和免疫学证据。
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人类白细胞抗原B∗27等位基因(HLA-B∗27)与脊柱关节炎及相关风湿病的密切关系一直令研究人员着迷,但其致病的确切机制却仍然难以捉摸。在这里,我们回顾了微生物组、免疫系统和神秘的 HLA-B∗27 之间的相互作用是如何诱发脊柱关节炎的,重点是 HLA-B∗27 是否会产生致关节炎肽。我们提出了 HLA-B∗27 蛋白结构的独特生化特性(尤其是其肽结合槽)可能决定其诱导病理 T 细胞反应倾向的机制。我们进一步提供了关于 TRBV9+ CD8+ T 细胞如何参与疾病过程以及 T 细胞的免疫代谢如何调节脊柱关节炎组织特异性炎症反应的新见解。最后,鉴于计算生物学、化学生物学、结构生物学和小分子疗法的最新进展,我们提出了可检验的模型,并建议在未来研究中解决这一问题的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
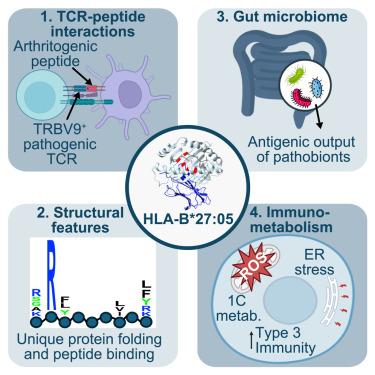

Emerging biochemical, microbial and immunological evidence in the search for why HLA-B∗27 confers risk for spondyloarthritis
The strong association of the human leukocyte antigen B∗27 alleles (HLA-B∗27) with spondyloarthritis and related rheumatic conditions has long fascinated researchers, yet the precise mechanisms underlying its pathogenicity remain elusive. Here, we review how interplay between the microbiome, the immune system, and the enigmatic HLA-B∗27 could trigger spondyloarthritis, with a focus on whether HLA-B∗27 presents an arthritogenic peptide. We propose mechanisms by which the unique biochemical characteristics of the HLA-B∗27 protein structure, particularly its peptide binding groove, could dictate its propensity to induce pathological T cell responses. We further provide new insights into how TRBV9+ CD8+ T cells are implicated in the disease process, as well as how the immunometabolism of T cells modulates tissue-specific inflammatory responses in spondyloarthritis. Finally, we present testable models and suggest approaches to this problem in future studies given recent advances in computational biology, chemical biology, structural biology, and small-molecule therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


