碘介导的脱羧偶联以 α-氨基酸和亚硫酸钠为原料合成 β-磺酰烯胺/2,3-二芳基吡咯。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种选择性合成 β-砜基烯胺和 2,3-二基硫代吡咯的新工艺。该工艺利用了 α- 氨基酸的脱羧偶联和 β-C(sp3)-H 功能化。在这一反应中,碘既可作为串联催化剂启动 α- 氨基酸的脱羧反应,又可作为氧化剂促进有机硫化物的形成。这种创新方法不仅简化了合成过程,还提高了所需产物的产量和选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
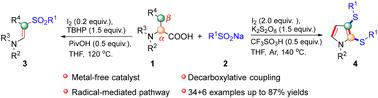
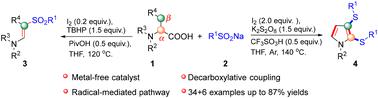
Iodine-mediated decarboxylative coupling to synthesize β-sulfonyl-ene-amines/2,3-diarythio-pyrroles from α-amino acids and sodium sulfinates†
A novel process has been developed for the selective synthesis of β-sulfonyl-enamines and 2,3-diarylthiopyrroles. This process utilizes the decarboxylative coupling and β-C(sp3)–H functionalization of α-amino acids. In this reaction, iodine functions dually as a tandem catalyst to initiate the decarboxylation of α-amino acids and as an oxidant to facilitate the formation of organic sulfides. This innovative approach not only simplifies the synthesis but also enhances the yield and selectivity of the desired products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


