肿瘤编辑会抑制先天性和适应性抗肿瘤免疫,而抑制 DNA 甲基化则可逆转这种情况
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
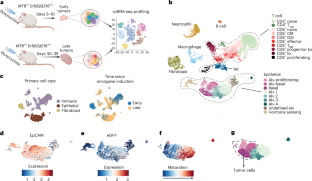
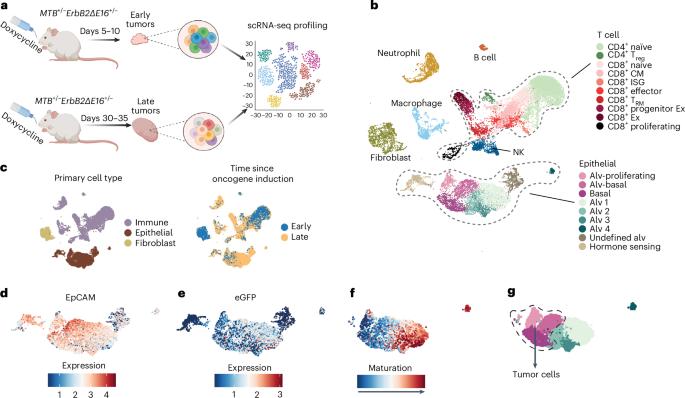
癌细胞编辑基因表达以逃避免疫监视。然而,目前还缺乏对早期肿瘤发生过程中基因编辑的全基因组研究。在这里,我们在乳腺癌基因工程小鼠模型(GEMM)中使用单细胞RNA测序技术,无偏差地识别编辑基因。晚期肿瘤抑制了抗肿瘤免疫基因,减少了浸润免疫细胞和肿瘤免疫细胞之间的交流。先天性免疫基因,尤其是干扰素刺激基因,在下调的肿瘤基因列表中占主导地位,而调控细胞内在恶性程度的基因大多未被编辑。早期肿瘤中的幼稚和活化的 CD8+ T 细胞被晚期肿瘤中的衰竭或前体衰竭细胞所取代。通过使用低剂量地西他滨抑制DNA甲基化,逆转了免疫基因的抑制,抑制了肿瘤生长,恢复了免疫控制,增加了肿瘤浸润淋巴细胞的数量、功能和记忆,减少了骨髓抑制细胞的数量。地西他滨诱导了 GEMM 和植入性乳腺癌和黑色素瘤的重要干扰素、热休克和坏死基因、炎性细胞死亡和免疫控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumor editing suppresses innate and adaptive antitumor immunity and is reversed by inhibiting DNA methylation
Cancer cells edit gene expression to evade immunosurveillance. However, genome-wide studies of gene editing during early tumorigenesis are lacking. Here we used single-cell RNA sequencing in a breast cancer genetically engineered mouse model (GEMM) to identify edited genes without bias. Late tumors repressed antitumor immunity genes, reducing infiltrating immune cells and tumor–immune cell communications. Innate immune genes, especially interferon-stimulated genes, dominated the list of downregulated tumor genes, while genes that regulate cell-intrinsic malignancy were mostly unedited. Naive and activated CD8+ T cells in early tumors were replaced with exhausted or precursor-exhausted cells in late tumors. Repression of immune genes was reversed by inhibiting DNA methylation using low-dose decitabine, which suppressed tumor growth and restored immune control, increasing the number, functionality and memory of tumor-infiltrating lymphocytes and reducing the number of myeloid suppressor cells. Decitabine induced important interferon, pyroptosis and necroptosis genes, inflammatory cell death and immune control in GEMM and implanted breast and melanoma tumors. Tumor immunoediting occurs early during tumorigenesis and is thereby difficult to study. Here the authors overcome this issue using an aggressive breast cancer model that enables them to compare and contrast the trancriptomic state of early versus late tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


