粘附素 RadD 可增强核酸镰刀菌的肿瘤定植和结肠直肠癌的发生
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
核叉杆菌能与宿主细胞结合并促进肠道肿瘤发生。在这里,我们利用全基因组筛选确定了一种粘附素 RadD,它能在体外促进 F. nucleatum 与结直肠癌(CRC)细胞的粘附。RadD直接与CD147结合,CD147是一种在CRC细胞表面过度表达的受体,它启动了PI3K-AKT-NF-κB-MMP9级联反应,随后增强了小鼠的肿瘤发生。临床标本分析表明,CRC 组织中 radD 基因水平的升高与激活的致癌信号和患者的不良预后呈正相关。最后,在小鼠体内阻断 RadD 与 CD147 之间的相互作用可有效抑制 F. nucleatum 的附着并减轻 F. nucleatum 诱导的致癌反应。总之,我们的研究深入揭示了由F. nucleatum RadD驱动的致癌机制,并表明RadD与CD147的相互作用可能是治疗CRC的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
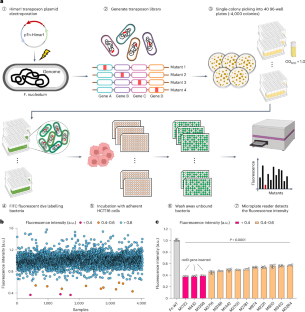
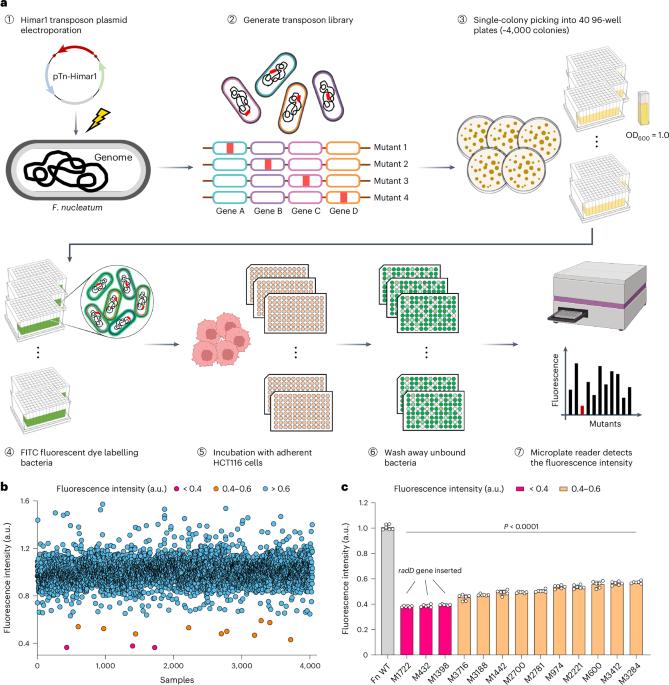
The adhesin RadD enhances Fusobacterium nucleatum tumour colonization and colorectal carcinogenesis
Fusobacterium nucleatum can bind to host cells and potentiate intestinal tumorigenesis. Here we used a genome-wide screen to identify an adhesin, RadD, which facilitates the attachment of F. nucleatum to colorectal cancer (CRC) cells in vitro. RadD directly binds to CD147, a receptor overexpressed on CRC cell surfaces, which initiated a PI3K–AKT–NF–κB–MMP9 cascade, subsequently enhancing tumorigenesis in mice. Clinical specimen analysis showed that elevated radD gene levels in CRC tissues correlated positively with activated oncogenic signalling and poor patient outcomes. Finally, blockade of the interaction between RadD and CD147 in mice effectively impaired F. nucleatum attachment and attenuated F. nucleatum-induced oncogenic response. Together, our study provides insights into an oncogenic mechanism driven by F. nucleatum RadD and suggests that the RadD–CD147 interaction could be a potential therapeutic target for CRC. The bacterial adhesin, RadD, enhances the ability of Fusobacterium nucleatum to interact with colorectal cancer cells and promote tumour development in mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


