核小体和甲基化 DNA 上 MeCP2 功能的差异动力学说明
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
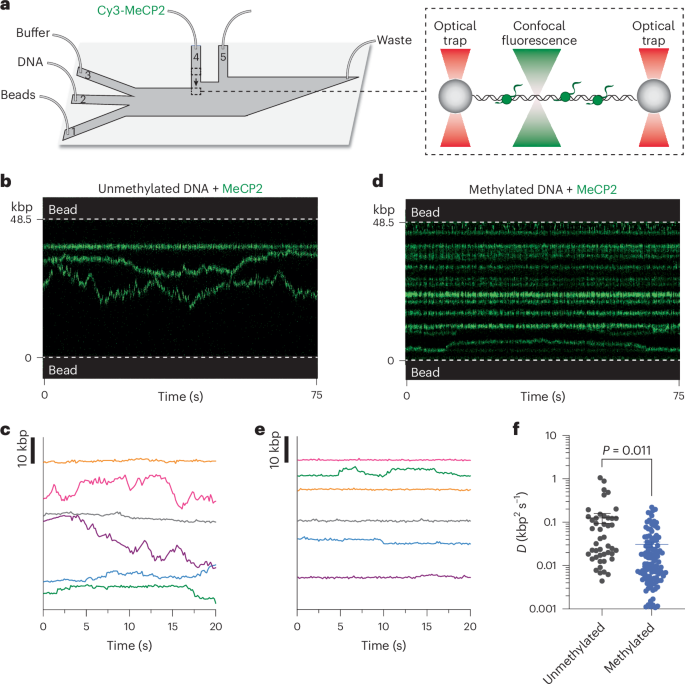
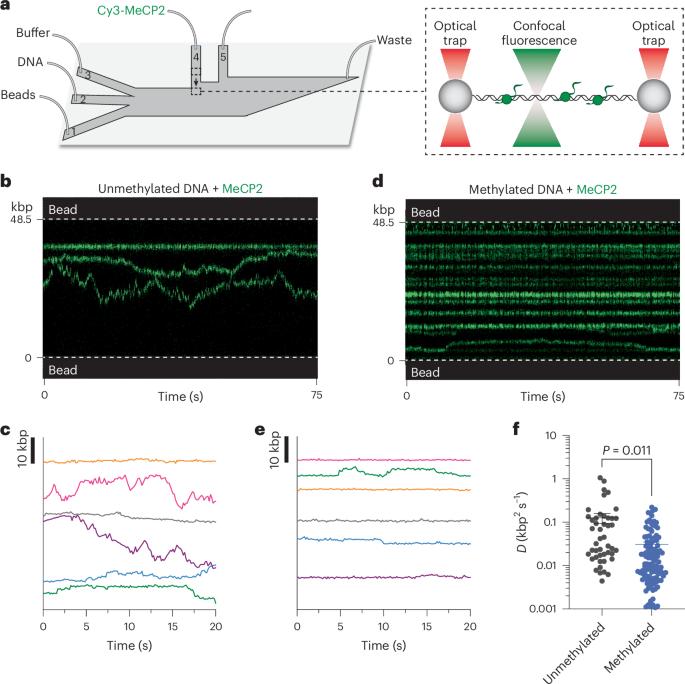
甲基-CpG结合蛋白2(MeCP2)是一种重要的染色质结合蛋白,其突变会导致雷特综合征(RTT),这是一种主要影响年轻女性的严重神经系统疾病。事实证明,将 MeCP2 视为 DNA 甲基化依赖性转录抑制因子的传统观点不足以描述它与染色质的动态相互作用以及在基因组组织和基因表达中的多方面作用。在这里,我们使用单分子相关力和荧光显微镜直接观察了野生型和RTT致突变体MeCP2在DNA上的动态。我们发现,当 MeCP2 与未甲基化 DNA 和 CpG 甲基化 DNA 结合时,会表现出不同的一维扩散动力学,从而实现甲基化特异性活动,如共抑制因子招募。我们还发现,在染色质化的 DNA 上,MeCP2 会优先定位于核小体,并使其免受机械扰动。我们的研究结果揭示了MeCP2在染色质上的多模式行为,这种行为是其DNA甲基化和核糖体依赖性功能的基础,并为剖析RTT突变的分子病理学提供了一个生物物理框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Differential dynamics specify MeCP2 function at nucleosomes and methylated DNA
Methyl-CpG-binding protein 2 (MeCP2) is an essential chromatin-binding protein whose mutations cause Rett syndrome (RTT), a severe neurological disorder that primarily affects young females. The canonical view of MeCP2 as a DNA methylation-dependent transcriptional repressor has proven insufficient to describe its dynamic interaction with chromatin and multifaceted roles in genome organization and gene expression. Here we used single-molecule correlative force and fluorescence microscopy to directly visualize the dynamics of wild-type and RTT-causing mutant MeCP2 on DNA. We discovered that MeCP2 exhibits distinct one-dimensional diffusion kinetics when bound to unmethylated versus CpG methylated DNA, enabling methylation-specific activities such as co-repressor recruitment. We further found that, on chromatinized DNA, MeCP2 preferentially localizes to nucleosomes and stabilizes them from mechanical perturbation. Our results reveal the multimodal behavior of MeCP2 on chromatin that underlies its DNA methylation- and nucleosome-dependent functions and provide a biophysical framework for dissecting the molecular pathology of RTT mutations. Using single-molecule techniques, the authors find that the methyl-CpG-binding protein MeCP2, whose mutations cause Rett syndrome, exhibits distinctive behaviors when bound to nucleosomes versus free DNA, thus directing its multifaceted functions on chromatin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


