原子分散的氢化钌在β沸石上作为短碳酸盐异构化的催化剂
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
寻找可持续聚合物需要获得基于生物质的单体。从这个意义上说,葡萄糖衍生的顺式、顺式粘多酸是一种极具潜力的中间体。然而,要释放其潜力,需要将其异构化为高附加值的反式、反式异构体--反式、反式粘多酸。在此,我们开发了原子分散低负载 Ru 的β沸石催化剂,可在乙醇中生产反式、反式粘多酸,总转化率(达到平衡)和选择性高达 95%。我们的单位 Ru 转化率非常高,生产率达到 427 mM h-1(约 85 g l-1 h-1),比生物基顺式、顺式粘多酸生产率高出一个数量级。通过将异构化与 Diels-Alder 环加成耦合,对苯二甲酸盐中间体的产量约为 90%,避开了异构体平衡。傅立叶变换红外光谱证明,Ru 氢化物可促进异构化,而氢化物是从醇溶剂中生成的。除了异构化,Ru 沸石及其氢化物形成能力还可用作其他氢化物化学的异质催化剂,氢化物转移加氢反应的成功就证明了这一点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
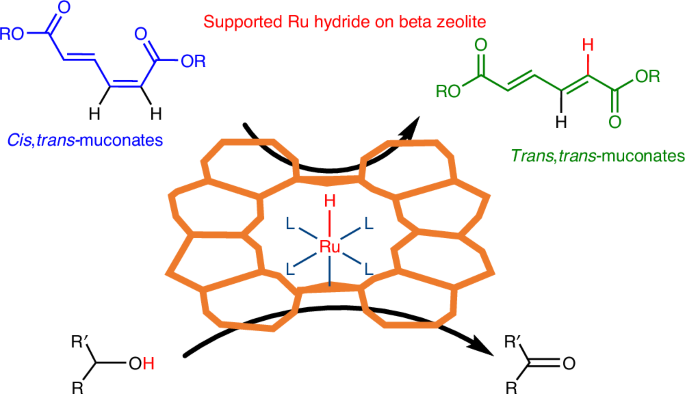
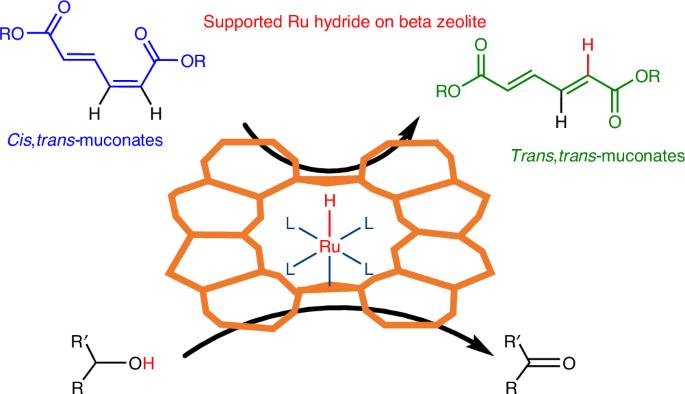
Atomically dispersed ruthenium hydride on beta zeolite as catalysts for the isomerization of muconates
Searching for sustainable polymers requires access to biomass-based monomers. In that sense, glucose-derived cis,cis-muconic acid stands as a high-potential intermediate. However, to unlock its potential, an isomerization to the value-added trans,trans-isomer, trans,trans-muconic acid, is required. Here we develop atomically dispersed low-loaded Ru on beta zeolite catalysts that produce trans,trans-muconate in ethanol with total conversion (to equilibrium) and a selectivity of >95%. We reach very high turnovers per Ru and productivity rates of 427 mM h−1 (~85 g l−1 h−1), surpassing the bio-based cis,cis-muconic acid production rates by an order of magnitude. By coupling isomerization to Diels–Alder cycloaddition, terephthalate intermediates are produced in around 90% yields, circumventing the isomer equilibrium. Isomerization is promoted by Ru hydride species where the hydrides are generated from the alcohol solvent, as evidenced by Fourier transform infrared spectroscopy. Beyond isomerization, the Ru–zeolite and its hydride-forming capacity could be of use as a heterogeneous catalyst for other hydride chemistries, demonstrated by a successful hydride transfer hydrogenation. Muconic acid is an important bio-based chemical; however its applications are limited by the lack of efficient methods to access its trans,trans-isomer. Here the authors address this problem with a catalyst based on single Ru atoms dispersed on zeolite BEA that is capable of unlocking hydride chemistries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


