层状硅的低温去合金化和独特的自填充界面优化机制可提高锂存储能力
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
层状硅(L-Si)阳极因其理论容量高而备受赞誉,但在制备过程中却面临着安全性和材料纯度方面的重大挑战。本研究采用 NH4Cl-CaSi2 作为原材料,通过蒸汽脱合金工艺解决了这些难题,与传统方法相比,该工艺提高了安全性和纯度。所制备的 L-Si 阳极具有出色的电化学性能,在 0.5 A g-¹ 的电流密度下可提供 1497.7 mAh g-¹ 的高可逆锂存储容量,并在 1200 次充放电循环中表现出稳定的性能。原位和非原位特性分析表明,电解质分解产物有效地填充了电极内部的空隙,而 L-Si 结构的逐渐分解有助于形成致密的导电网络。这一过程增强了锂离子传输,并利用了层状硅的电容存储优势。这项研究的见解为开发和优化其他硅基负极材料提供了宝贵的指导,为高性能锂离子电池的开发铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
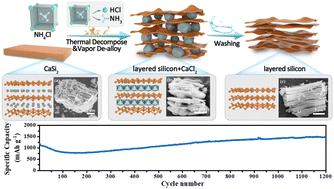
Low-temperature de-alloying and unique self-filling interface optimization mechanism of layered silicon for enhanced lithium storage†
Layered silicon (L–Si) anodes are celebrated for their high theoretical capacity but face significant challenges regarding safety and material purity during preparation. This study addresses these challenges by employing NH4Cl–CaSi2 as the raw material in a gas-solid de-alloying process, which enhances both safety and purity compared to traditional methods. The L–Si anodes produced demonstrate outstanding electrochemical performance, delivering a high reversible lithium storage capacity of 1497.7 mA h g−1 at a current density of 0.5 A g−1, and exhibiting stable performance over 1200 charge–discharge cycles. In situ and ex situ characterizations reveal that electrolyte decomposition products effectively fill the voids within the electrode, while the gradual disintegration of the L–Si structure contributes to the formation of a dense, conductive network. This process enhances lithium ion transport and exploits the capacitive storage benefits of layered silicon.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


