在叶绿体相关蛋白降解过程中,UBX 域蛋白将 Cdc48 招募到叶绿体中
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
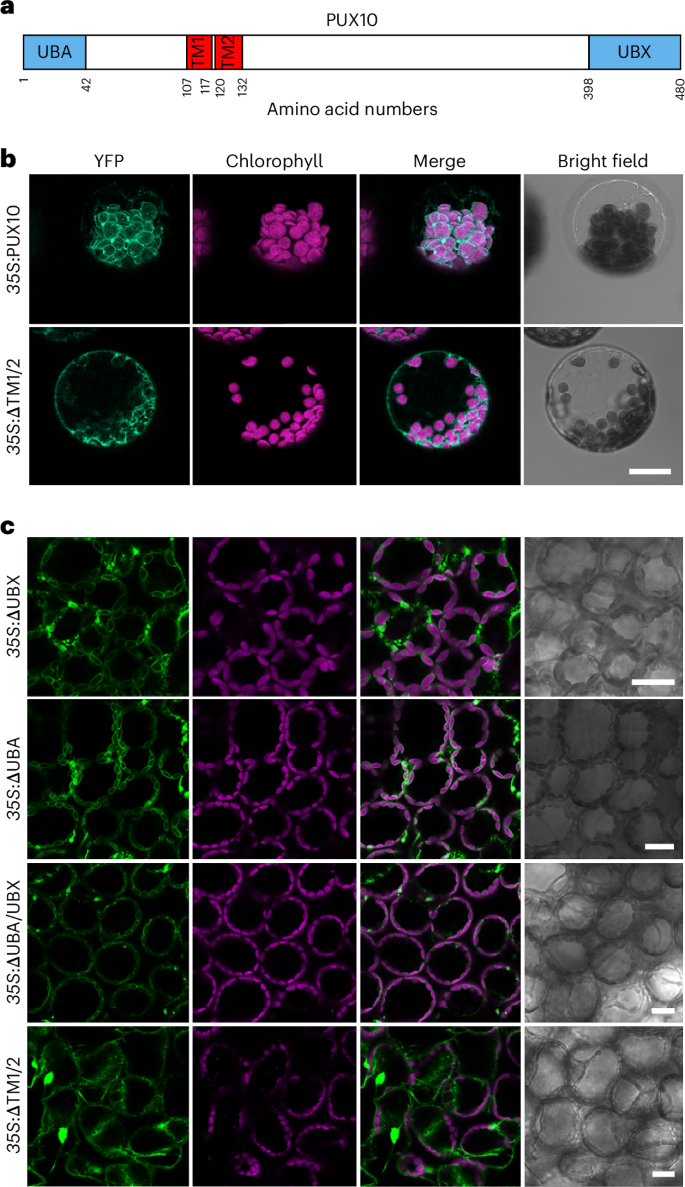
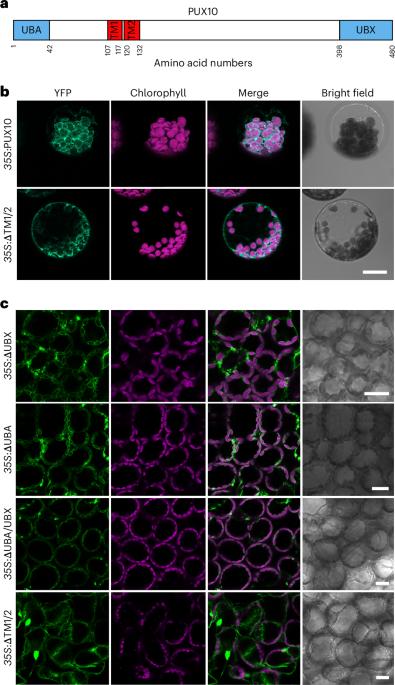
叶绿体外膜上的转化子(TOC)是叶绿体蛋白质输入的门户,因此对光合作用的建立和植物生长至关重要。叶绿体相关蛋白降解(CHLORAD)是一种依赖于泛素的蛋白水解系统,可调节 TOC。在 CHLORAD 中,细胞质 Cdc48 为泛素化 TOC 蛋白向细胞质逆转位提供动力,但 Cdc48 是如何被招募的尚不清楚。在这里,我们发现植物 UBX 域蛋白 PUX10 是 CHLORAD 机制的一个组成部分。我们发现,PUX10 是一种完整的叶绿体外膜蛋白,能将 UBX 和泛素相关结构域投射到细胞质中。它通过其 UBX 结构域与 Cdc48 相互作用,将其带到叶绿体表面,并通过其泛素相关结构域与泛素化的 TOC 蛋白相互作用。拟南芥的遗传分析表明,在 CHLORAD 介导的 TOC 功能调控和植物发育过程中需要 PUX10。因此,PUX10 可协调 CHLORAD 的泛素化和逆转位活动,从而实现高效的 TOC 转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Recruitment of Cdc48 to chloroplasts by a UBX-domain protein in chloroplast-associated protein degradation
The translocon at the outer chloroplast membrane (TOC) is the gateway for chloroplast protein import and so is vital for photosynthetic establishment and plant growth. Chloroplast-associated protein degradation (CHLORAD) is a ubiquitin-dependent proteolytic system that regulates TOC. In CHLORAD, cytosolic Cdc48 provides motive force for the retrotranslocation of ubiquitinated TOC proteins to the cytosol but how Cdc48 is recruited is unknown. Here, we identify plant UBX-domain protein PUX10 as a component of the CHLORAD machinery. We show that PUX10 is an integral chloroplast outer membrane protein that projects UBX and ubiquitin-associated domains into the cytosol. It interacts with Cdc48 via its UBX domain, bringing it to the chloroplast surface, and with ubiquitinated TOC proteins via its ubiquitin-associated domain. Genetic analyses in Arabidopsis revealed a requirement for PUX10 during CHLORAD-mediated regulation of TOC function and plant development. Thus, PUX10 coordinates ubiquitination and retrotranslocation activities of CHLORAD to enable efficient TOC turnover. Extraction of ubiquitinated proteins from chloroplasts in CHLORAD is driven by the cytosolic ATPase Cdc48. The UBX-domain protein PUX10 is shown to be a CHLORAD component that recruits Cdc48 to the chloroplast surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


