合成多肽通过破坏多价 TLR9 与 LL37-DNA 束的结合,抑制核酸诱导的自身免疫性疾病炎症
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
细胞外核酸(NAs)与内源性蛋白质或肽的复合物(如 LL37)会打破免疫平衡并引发自身免疫性疾病,而与富含精氨酸的肽的复合物则不会。受此启发,我们合成了一种多精氨酸纳米粒子 PEG-TK-NPArg,与 LL37 相反,它能有效抑制 Toll 样受体-9(TLR9)的激活。为了探索PEG-TK-NPArg和LL37的差异效应,我们利用小角X射线散射评估了PEG-TK-NPArg-NA和LL37-NA复合物的周期性结构。LL37-NA复合物的NA间距较大,可容纳TLR9,而PEG-TK-NPArg-NA复合物的NA间距与TLR9的空腔错配,从而抑制了与多个TLR9的相互作用,限制了它们的聚集,抑制了免疫诱导。随后,PEG-TK-NPArg 的抑制炎症作用在类风湿性关节炎动物模型中得到了证实。这项关于清道夫-NA 复合物如何抑制免疫反应的研究有助于将概念验证研究转化为临床应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
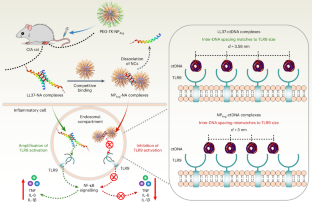
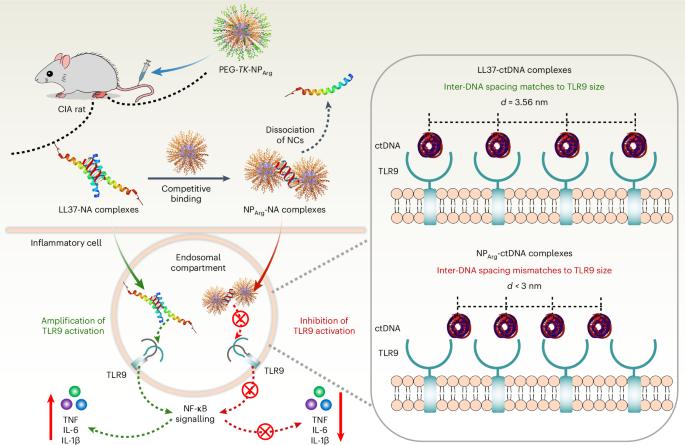
Synthetic polypeptides inhibit nucleic acid-induced inflammation in autoimmune diseases by disrupting multivalent TLR9 binding to LL37-DNA bundles
Complexes of extracellular nucleic acids (NAs) with endogenous proteins or peptides, such as LL37, break immune balance and cause autoimmune diseases, whereas NAs with arginine-enriched peptides do not. Inspired by this, we synthesize a polyarginine nanoparticle PEG-TK-NPArg, which effectively inhibits Toll-like receptor-9 (TLR9) activation, in contrast to LL37. To explore the discrepancy effect of PEG-TK-NPArg and LL37, we evaluate the periodic structure of PEG-TK-NPArg-NA and LL37-NA complexes using small-angle X-ray scattering. LL37-NA complexes have a larger inter-NA spacing that accommodates TLR9, while the inter-NA spacing in PEG-TK-NPArg-NA complexes mismatches with the cavity of TLR9, thus inhibiting an interaction with multiple TLR9s, limiting their clustering and damping immune induction. Subsequently, the inhibitory inflammation effect of PEG-TK-NPArg is proved in an animal model of rheumatoid arthritis. This work on how the scavenger-NA complexes inhibit the immune response may facilitate proof-of-concept research translating to clinical application. This study shows how amino acid composition and topology in synthetic polypeptides affect anti-inflammatory effects and how scavenging debris nucleic acids inhibits inflammation and relieves symptoms of autoimmune diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


