高脂肪饮食对代谢健康的影响因绝经年龄而异。
IF 3.8
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
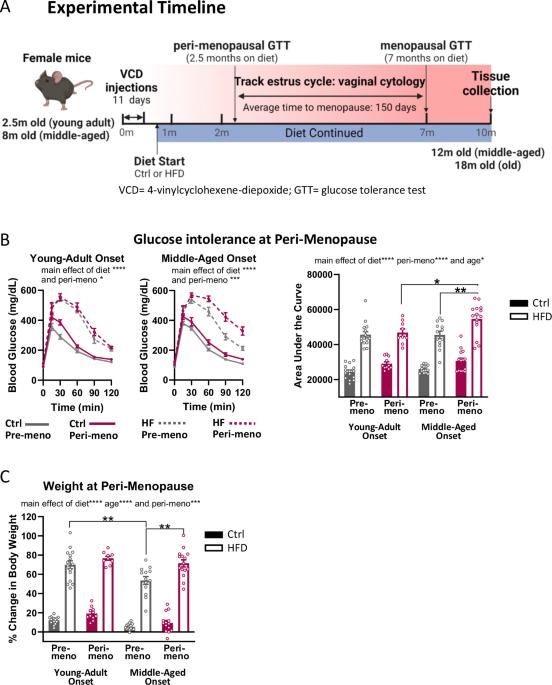
更年期会加速代谢功能障碍,包括(前期)糖尿病、肥胖和内脏脂肪过多。然而,人们对内分泌衰老与时间衰老在这一过程中的影响还知之甚少。我们假设,更年期,尤其是中年更年期,会加剧高脂肪饮食对新陈代谢的影响。我们利用年轻成年和中年的 C57BL/6J 雌性小鼠,通过长期摄入高脂肪(HF)饮食与对照饮食的对比,建立了饮食诱导肥胖的模型。我们通过注射会加速卵巢功能衰竭的 4-乙烯基环己烯二环氧化物与药物,模拟了围绝经期/更年期。我们分别在围绝经期和绝经期开始节食后 2.5 个月和 7 个月进行了葡萄糖耐量测试。围绝经期增加了中年高密度脂蛋白喂养小鼠葡萄糖不耐受的严重程度和体重增加。更年期会增加所有小鼠的体重增加,与年龄和饮食无关,而慢性衰老会促使脂肪组织分布发生变化,内脏脂肪增加,皮下脂肪减少。这些数据与临床数据相符,临床数据显示绝经后妇女更容易出现代谢功能障碍,并表明更大的计时年龄会加剧内分泌衰老(绝经)的影响。这项工作强调了在代谢健康研究中同时考虑计时衰老和内分泌衰老的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effects of high fat diet on metabolic health vary by age of menopause onset
Menopause accelerates metabolic dysfunction, including (pre-)diabetes, obesity and visceral adiposity. However, the effects of endocrine vs. chronological aging in this progression are poorly understood. We hypothesized that menopause, especially in the context of middle-age, would exacerbate the metabolic effects of a high fat diet. Using young-adult and middle-aged C57BL/6J female mice, we modeled diet-induced obesity via chronic administration of high fat (HF) diet vs. control diet. We modeled peri-menopause/menopause via injections of 4-vinylcyclohexene diepoxide, which accelerates ovarian failure vs. vehicle. We performed glucose tolerance tests 2.5 and 7 months after diet onset, during the peri-menopausal and menopausal phases, respectively. Peri-menopause increased the severity of glucose intolerance and weight gain in middle-aged, HF-fed mice. Menopause increased weight gain in all mice regardless of age and diet, while chronological aging drove changes in adipose tissue distribution towards more visceral vs. subcutaneous adiposity. These data are in line with clinical data showing that post-menopausal women are more susceptible to metabolic dysfunction and suggest that greater chronological age exacerbates the effects of endocrine aging (menopause). This work highlights the importance of considering both chronological and endocrine aging in studies of metabolic health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Obesity
医学-内分泌学与代谢
CiteScore
10.00
自引率
2.00%
发文量
221
审稿时长
3 months
期刊介绍:
The International Journal of Obesity is a multi-disciplinary forum for research describing basic, clinical and applied studies in biochemistry, physiology, genetics and nutrition, molecular, metabolic, psychological and epidemiological aspects of obesity and related disorders.
We publish a range of content types including original research articles, technical reports, reviews, correspondence and brief communications that elaborate on significant advances in the field and cover topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


