通过镍催化的阿伦炔环二聚化合成梯烷:范围和机理
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
我们开发了一种镍催化的 1,6-烯烃环二聚化反应,可生成含有 [3]- 梯烷的五环衍生物。在一次操作中就能形成六个新的碳-碳键,完全符合原子经济学原理。该反应在廉价催化剂的作用下进行,并在炔烃、烯烃和系链基团中显示出广泛的应用范围。对反应机理的计算和实验研究表明,这一过程是通过与 Ni(0) 配位的烯烃发生氧化环金属化反应,然后进行还原消除反应,生成环丁烯。然后,其中两个分子按照相关的环金属化-还原消除顺序耦合,后者是限速步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
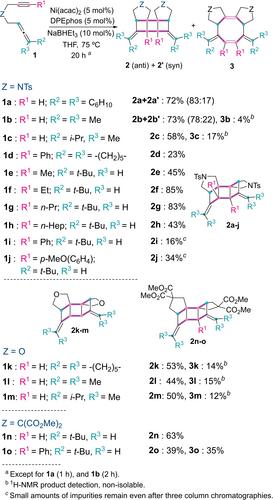
Synthesis of Ladderanes by Nickel‐Catalyzed Cyclodimerization of Allenynes: Scope and Mechanism
We have developed a Ni‐catalyzed cyclodimerization of 1,6‐allenyenes which affords pentacyclic derivatives containing a [3]‐ladderane. Six new carbon‐carbon bonds are formed in a single operation which is fully atom‐economical. The reaction proceeds with an inexpensive catalyst and shows a broad scope in the alkyne, the allene and the tethering group. A computational and experimental study on the reaction mechanism suggests that this process proceeds by oxidative cyclometallation of the allenyne coordinated to Ni(0) followed by reductive elimination leading to cyclobutenes. Then, two of these molecules are coupled following a related cyclometallation‐reductive elimination sequence, the latter being the rate‐limiting step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


