非水氧化碱介导的电化学碳捕获
IF 60.1
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
利用氧化还原可调的有机吸附剂进行电化学介导的碳捕获已成为减缓人为二氧化碳排放的一种有前途的策略。然而,大多数报道的系统对分子氧敏感,严重限制了它们在环境空气条件下的应用。在这里,我们展示了一种通过非水质质子耦合电子转移进行电化学碳捕获的概念,其中烷氧基化合物被用作活性吸附剂,而二氧化碳的吸收和解吸则由某些有机分子的氧化还原可调布氏碱性可逆调节。由于该过程中涉及的所有物质都具有出色的氧稳定性和相对较低的蒸汽压,我们的电化学介导碳捕集机制从本质上最大限度地减少了有氧条件下的寄生反应和蒸发损失。基于流动的原型已证明可在 20% 的氧气存在下,在各种实际相关的二氧化碳进料浓度下高效运行,为电化学刺激驱动的有效碳捕获铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
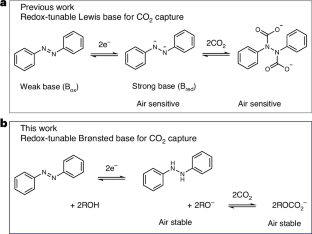
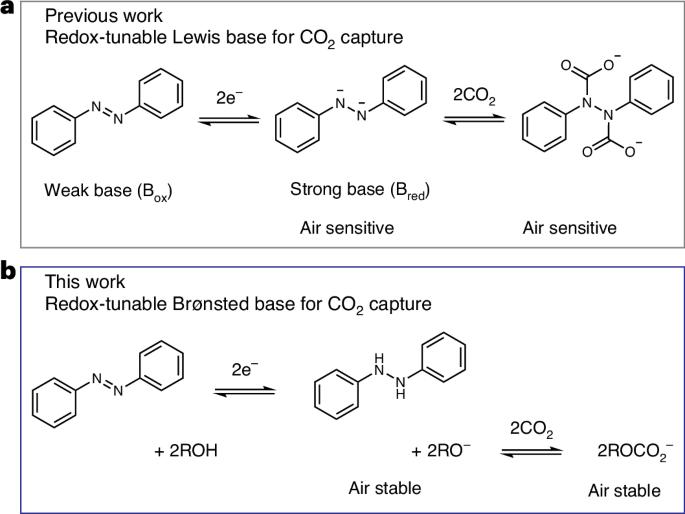
Non-aqueous alkoxide-mediated electrochemical carbon capture
Electrochemically mediated carbon capture utilizing redox-tunable organic sorbents has emerged as a promising strategy to mitigate anthropogenic carbon dioxide emissions. However, most reported systems are sensitive to molecular oxygen, severely limiting their application under ambient air conditions. Here we demonstrate an electrochemical carbon capture concept via non-aqueous proton-coupled electron transfer, where alkoxides are employed as the active sorbent while carbon dioxide absorption and desorption are modulated reversibly by the redox-tunable Brønsted basicity of certain organic molecules. Since all species involved in the process have outstanding oxygen stability and relatively low vapour pressure, our electrochemically mediated carbon capture mechanism intrinsically minimizes parasitic reactions and evaporative losses under aerobic conditions. Flow-based prototypes are demonstrated to operate efficiently in the presence of 20% oxygen under various practically relevant carbon dioxide feed concentrations, paving a way towards effective carbon capture driven by electrochemical stimuli. Sensitivity to O2 hinders the application of some electrochemically mediated carbon capture technologies under ambient air conditions. Here the authors report electrochemical CO2 capture via non-aqueous proton-coupled electron transfer, employing alkoxides as active sorbents, in which all species involved are highly air stable.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


