基于活性的元蛋白质组学发现小鼠肠道微生物组中潜在的α-半乳糖苷酶并对其进行酶学鉴定
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
肠道微生物群提供了广泛的酶资源,但许多酶仍未定性。为了区分类似注释蛋白质的活性并挖掘微生物群中潜在的适用蛋白质,我们采用了一种有效的基于活性的元蛋白质组学(ABMP)策略,使用特定的基于活性的探针(ABP)筛选整个肠道微生物群,直接发现活性酶及其潜在应用,而不是探索宿主与微生物群之间的相互作用。通过使用特异于α-半乳糖苷酶(AGAL)的基于活性的环黄酮氮丙啶探针,我们成功鉴定并描述了几种具有AGAL活性的肠道微生物群酶。对一种新鉴定酶(AGLA5)的冷冻电镜分析揭示了AGAL5活性位点与环黄酮氮丙啶ABP之间的共价结合构象,这有助于深入了解该酶的催化机理。这四种新表征的 AGALs 具有多种潜在活性,包括棉子糖家族低聚糖(RFO)水解和酶促血型转化。总之,我们提出了一个 ABMP 平台,该平台有助于肠道微生物群 AGALs 的发现、生化活性注释以及潜在的工业或生物制药应用。肠道微生物群提供了大量的酶资源,但许多酶仍未定性。在本文中,作者采用了一种基于活性的元蛋白质组学策略,使用一种特异于α-半乳糖苷酶(AGAL)的基于活性的环黄酮氮丙啶探针,鉴定并描述了几种具有AGAL活性的肠道微生物群酶。本文章由计算机程序翻译,如有差异,请以英文原文为准。
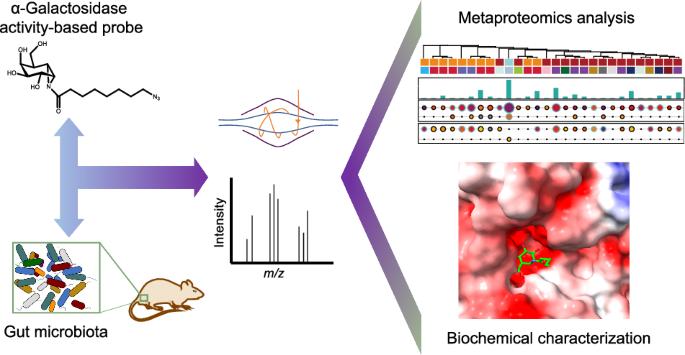
Activity-based metaproteomics driven discovery and enzymological characterization of potential α-galactosidases in the mouse gut microbiome
The gut microbiota offers an extensive resource of enzymes, but many remain uncharacterized. To distinguish the activities of similar annotated proteins and mine the potentially applicable ones in the microbiome, we applied an effective Activity-Based Metaproteomics (ABMP) strategy using a specific activity-based probe (ABP) to screen the entire gut microbiome for directly discovering active enzymes and their potential applications, not for exploring host-microbiome interactions. By using an activity-based cyclophellitol aziridine probe specific to α-galactosidases (AGAL), we successfully identified and characterized several gut microbiota enzymes possessing AGAL activities. Cryo-electron microscopy analysis of a newly characterized enzyme (AGLA5) revealed the covalent binding conformations between the AGAL5 active site and the cyclophellitol aziridine ABP, which could provide insights into the enzyme’s catalytic mechanism. The four newly characterized AGALs have diverse potential activities, including raffinose family oligosaccharides (RFOs) hydrolysis and enzymatic blood group transformation. Collectively, we present a ABMP platform that facilitates gut microbiota AGALs discovery, biochemical activity annotations and potential industrial or biopharmaceutical applications. The gut microbiota offers an extensive resource of enzymes, however, many remain uncharacterized. Here, the authors apply an activity-based metaproteomics strategy using an activity-based cyclophellitol aziridine probe specific to α-galactosidases (AGAL), and identify and characterize several gut microbiota enzymes possessing AGAL activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


