人类结直肠癌的单细胞综合分析揭示了具有不同免疫逃避机制的患者分层。
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
肿瘤微环境(TME)在很大程度上影响着结直肠癌(CRC)的进展、治疗反应和临床预后,但目前还缺乏对 CRC 中肿瘤微环境个体间异质性的研究。在这里,通过整合来自约 200 名供体的人类结直肠单细胞转录组数据,我们全面描述了 TME 与非癌症组织相比的转录重塑特征,并鉴定了具有 T 细胞招募潜能的罕见肿瘤特异性内皮细胞亚群。庞大的样本量使我们能够根据 TME 的异质性对患者进行分层,揭示出不同的 TME 亚型,在这些亚型中,癌细胞利用了不同的免疫逃避机制。此外,通过将单细胞转录谱分析与全基因组关联研究确定的风险基因联系起来,我们确定基质细胞是 CRC 遗传易感性的主要效应细胞类型。总之,我们的研究结果为了解 CRC 的发病机制提供了宝贵的信息,并可能有助于开发个性化的免疫疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
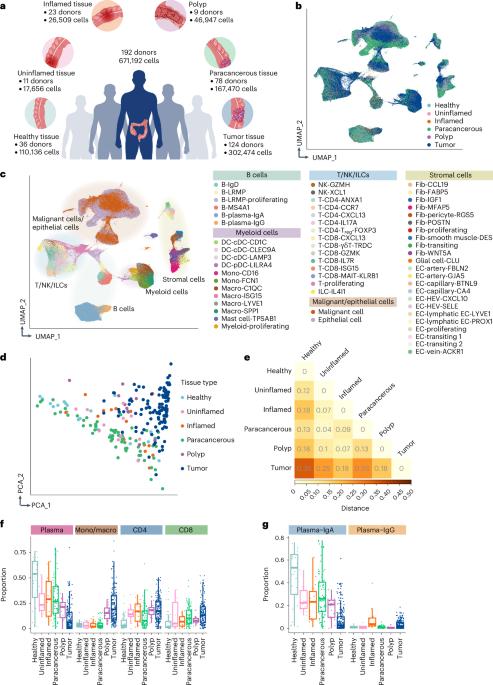
Integrative single-cell analysis of human colorectal cancer reveals patient stratification with distinct immune evasion mechanisms
The tumor microenvironment (TME) considerably influences colorectal cancer (CRC) progression, therapeutic response and clinical outcome, but studies of interindividual heterogeneities of the TME in CRC are lacking. Here, by integrating human colorectal single-cell transcriptomic data from approximately 200 donors, we comprehensively characterized transcriptional remodeling in the TME compared to noncancer tissues and identified a rare tumor-specific subset of endothelial cells with T cell recruitment potential. The large sample size enabled us to stratify patients based on their TME heterogeneity, revealing divergent TME subtypes in which cancer cells exploit different immune evasion mechanisms. Additionally, by associating single-cell transcriptional profiling with risk genes identified by genome-wide association studies, we determined that stromal cells are major effector cell types in CRC genetic susceptibility. In summary, our results provide valuable insights into CRC pathogenesis and might help with the development of personalized immune therapies. Cheng and colleagues performed an integrative analysis of human colorectal cancer samples to characterize the tumor microenvironment (TME) and stratify patients according to their heterogeneous TMEs, which exploit different immune evasion mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


