五对带相反电荷的聚电解质的骨架化学和离子官能团对络合物凝聚的影响。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
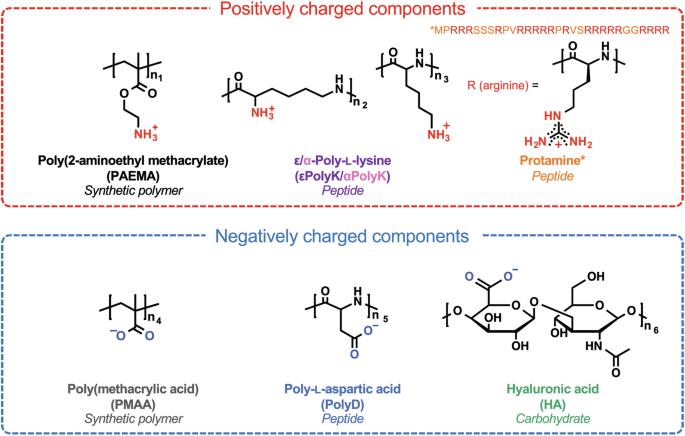
络合物共保在多个领域发挥着重要作用。本文研究了五对带相反电荷的聚电解质的骨架化学和离子官能团对络合物凝聚的影响。这五对聚电解质包括具有脂肪烃骨架的合成聚合物、具有酰胺键的肽和具有糖苷键的碳水化合物。尽管共用相同的带电基团,但根据聚电解质混合比、缓冲液和离子强度的不同,特定配对显示出不同的液相/液相和液相/固相分离。共凝胶的相界按照糖苷键>酰胺骨架>脂肪烃骨架,以及磷酸三酯>醋酸三酯>氯化物三酯缓冲液的顺序扩大。用在水中溶解度较低的聚电解质制备的凝聚体在高浓度盐溶液中不易分解,而且其合并速度较慢。这些观察结果表明,聚电解质中的疏水片段会干扰复合凝聚态的形成;然而,凝聚态形成后,疏水片段会使凝聚态变得稳定而富有弹性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of the backbone chemistry and ionic functional groups of five pairs of oppositely charged polyelectrolytes on complex coacervation
Complex coacervation plays an important role in various fields. Here, the influences of the backbone chemistry and ionic functional groups of five pairs of oppositely charged polyelectrolytes on complex coacervation were investigated. These pairs include synthetic polymers with aliphatic hydrocarbon backbones, peptides with amide bonds, and carbohydrates with glycosidic linkages. Despite sharing identical charged groups, specific pairs displayed distinct liquid/liquid and liquid/solid phase separations depending on the polyelectrolyte mixing ratio, buffer, and ionic strength. The coacervate phase boundary broadened in the orders: glycosidic linkages > amide backbone > aliphatic hydrocarbon backbone, and Tris-phosphate > Tris-acetate > Tris-chloride buffers. Coacervates prepared from polyelectrolytes with lower solubilities in water resisted disassembly at high salt concentrations, and their merge rate was slow. These observations suggest that the hydrophobic segments in polyelectrolytes interfere with the formation of complex coacervates; however, following coacervate formation, the hydrophobic segments render the coacervates stable and elastic. Complex coacervation is propelled by the electrostatic association between oppositely charged polyelectrolytes, but the factors that drive complex coacervation have yet to be fully understood. Here, the authors investigate the influence of the backbone chemistry and ionic functional groups of five pairs of oppositely charged polyelectrolytes on complex coacervation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


